1. Which one of the following methods is used to separate a mixture of diesel and water?
A. Filtration
B. Evaporation
C. Chromatography
D. Separating funnel
2. Which one of the following is the major constituent of air?
A. Oxygen
B. Nitrogen
C. Carbon dioxide
D. Water vapour
3. Which one of the following ions if present in water causes hardness?
A. Polyethene
B. Perspex
C. Nylon
D. Rubber
4. Which one of the following ions if present in water causes hardness>
A. Na+
B. Al3+
C. Mg2+
D. NH4 +
5. Which one of the following gases can be identified through smell?
A. H2S
B. CO2
C. HCl
D. O2
6. Which one of the following electronic configuration is of a noble gas?
A. 2:8:1
B. 2:8:1
C. 2:8:2
D. 2:8:7
7. Ammonia is not used
A. as fertilizer
B. as refridgerant
C. for reducing copper(II) oxide to copper
D. in the manufacture of nitric acid
8. Which one of the following metals will react most readily with cold water?
A. Sodium
B. Calcium
C. Magnesium
D. Potassium
9. Which one of the following substances when heated undergoes a chemical change?
A. Ammonium chloride
B. Copper (II) hydroxide
C. Candle wax
D. Sulphur
10. Which one of the following reagents is normally used to test for the presence of chloride ion in solution?
A. Potassium iodide
B. Barium nitrate
C. Silver nitrate
D. Lead(II) nitrate
11. Which one of the following substances is used to test for the presence of oxygen?
A. A glowing splint
B. A burning splint
C. Litmus paper
D. Anhydrous copper (II) sulphate
12. Which one of the following methods is used to separate the alkanes in crude petroleum?
A. Filtration
B. Decantation
C. Fractional distillation
D. Fractional crystallization.
13. Which one of the following will be the colour of the precipitate formed when lead(II) nitrate solution is added to sodium chloride solution?
A. Blue
B. Brown
C. Yellow
D. White
14. Which one of the following particles conducts electric current in molten lead(II)bromide?
A. Electrons
B. Molecules
C. Atoms
D. Ions
15. Which one of the following anions reacts with silver ions in solution to form precipitate that dissolves in aqueous ammonia solution?
A. SO4 2 (aq)
B. Cl- (aq)
C. CO32-(aq)
D. NO3 – (aq)
16. The electronic configurations of elements L,M, V and R are 2:8:3, 2:8:6, 2:8:8 and 2:8:8:2 respectively. Which one of the folloeing pairs of elements consists of metals only?
A. M and V
B. L and V
C. M and R
D. L and R
17. Which one of the following reactions of hydrochloric acid is an example of neutralization reaction? Reaction with
A. Zinc
B. Sodium sulphate
C. Magnesium oxide
D. Silver nitrate
18. Which one of the following is the number of moles of hydrogen ions in 100cm3 of a 0.05M sulphuric acid?
A. 0.0025
B. 0.01
C. 0.25
D. 1.00
19. Which one of the following gases will produce white fumes when placed near concentrated ammonia?
A. Hydrogen chloride
B. Sulphur dioxide
C. Hydrogen
D. Oxygen
20. When 16.3g of substance R were burnt in air, the heat produced raised the temperature of 200g of water by x0C. Which one of the following is the value of x?
[Molar heat of combustion of R = 355.5 kJ mol-1;
Relative atomic mass of R = 64;
Specific heat capacity of water = 4.2 J g-1 K-1]
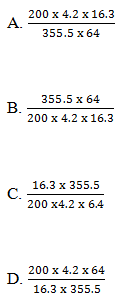
21. Which one of the following carbonates when heated strongly will decompose to form a blusih –black residue?
A. Pb CO3
B. MgCO3
C. ZnCO3
D. FeCO3
22. Which one of the following is the molarity of a 25.0cm3 sodium carbonate solution required to neutralize 20.0cm3 of a 0.15M dibasic acid?
A. 0.060M
B. 0.120M
C. 0.188M
D. 0.240M
23.Which one of the following compounds will turn bromine from reddish-brown to colourless?
A. C4H10
B. C3H8
C. C2H4
D. CH4
24. Which one of the following hydroxides will dissolve in ammonia solution?
A. Zn(OH)2
B. Al(OH)3
C. Pb(OH)2
D. Fe(OH)3
25. Element M reacts with dilute acids and forms a brown precipitate when added to copper(II) sulphate solution. Which one of the following is the order of reactivity of M, hydrogen and copper, starting with the most reactive?
A. Hydrogen > M> copper
B. M> copper> hydrogen
C. Copper>hydrogen>M
D. M>hydrogen>copper
26. A hydrocarbon CxHy burns in oxygen according to following equation;
CxHy(g) + 5O2(g) ---> 3CO2(g) + 4H20(l)
Which one of the following are the values of x and y respectively?
A. 1and 4
B. 2 and 4
C. 3 and 8
D. 4 and 10
27. Which of the following cations will react with dilute sodium hydroxide to form a precipitate that does not dissolve in excess alkali?
A. Al3+
B. Mg2+
C.Zn2+
D.Pb2+
28.Phosphoric acid can react with sodium hydroxide according to the following to the following equation.
3NaOH(aq) + H3PO4(aq) ---> Na3PO4(aq) + 3H2O(l)
The volume of a 0.1M phosphoric acid required to react completely with 30cm3 of a 0.2M sodium hydroxide solution is
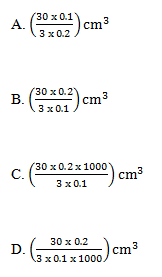
29. Which one of the following substances when burnt in oxygen will form product(s) that dissolve in water to give a solution with pH greater than 7?
A. Carbon
B. Ammonia
C. Sulphur
D. Calcium
30. Zinc carbonate was heated and the residue allowed to cool. Which one of the following is the colour of the residue?
A. Black
B. Yellow
C. White
D. Reddish-brown
31. Ammonia reacts with lead(II)oxide according to the following equation.
2NH3(g) + 3 PbO(s) ---> N2(g) + 3 Pb(s) + 3H2O(l)
Which one of the following is the volume of ammonia measured at s.t.p that would be required to react completely with 3.3g of lead(II)oxide?
[O = 16; Pb =207; 1 mo;e of gas occupies 22.4dm3 at room temperature]
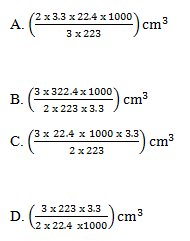
32. The atomic number of element X is 11. Which one of the following is not a property of the oxide of X?
A. It has a high melting point
B. It conducts electricity in solid state
C. It is soluble in water
D. It is a basic oxide
33. Butane burns in air according to the following equation;
2C4H10(g) + 13O2(g) ---> 8CO2(g) + 10H2O(l)
Which one of the following would be the mass of butane that would burn to produce 1150kJ of heat?
[H = 1; C= 12; Molar enthalpy of combustion of butane = -2877kJ mol-1]
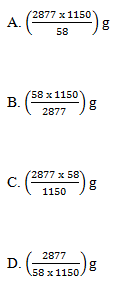
34. Which one of the following statements is correct about graphite and diamond? They both
A. have giant structures
B. Have similar physical properties
C. have different chemical properties
D. are very hard substances
35. Magnesium reacts with dilute hydrochloric acid according to the following equation.
Mg(s) + 2 HCl(aq) ---> MgCl2(aq) + H2(g)
Which one of the following is the volume of a 1.5 M hydrochloric acid that would react completely with 1.2g of magnesium? [Mg = 24]
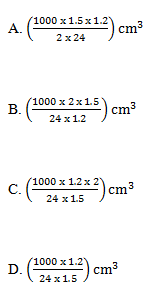
36. Sodium carbonate reacts with hydrochloric acid according to the following ionic equation.
CO32- (aq) + 2H+ (aq) ---> CO2(g) + H2O(l)
If 25.0cm3 of a 0.1M hydrochloric acid reacted completely with 17.8cm3 of a sodium carbonate solution, the concentration in moles per dm3 of the sodium carbonate solution was
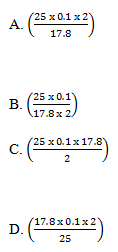
37. Which one of the following substances is not formed when zinc nitrate is heated strongly?
A. O2
B. ZnO
C. NO2
D. NO
38. Element Y has atomic number 13. The chemical bond in the sulphide of Y is
A. ionic bond
B. covalent bond
C. dative bond
D. metallic bond
39. Sulphur dioxide when exploded with oxygen, reacts to form sulphur trioxide according to the following equation:
2SO2(g) + O2(g) --> 2SO3(g)
If 10cm3 of sulphur dioxide were exploded with 15cm3 of oxygen and the resultant gas cooled to room temperature, the volume of the resultant gas would be
A. 25cm3
B. 15cm3
C. 10cm3
D. 5cm3
40. Which one of the following ions will produce a white precipitate with acidified barium nitrate solution?
A. Cl-
B. SO42-
C. CO32-
D. HCO3-
Each of the questions 41 to 45 consists of an assertion (statement) on the left hand side and a reason on the right-hand side
Select:
A: if both the assertion and the reason are true statements and the reason is a correct explanation of the assertion.
B: if both the assertion and the reason are true statements but the reason is not a correct explanation of the assertion
C: if the assertion is true but the reason is not a correct statement
D: if the assertion is not correct but the reason is a correct statement.
INSTRUCTIONS SUMMARISED:
|
Assertion |
Reason |
|
A. True |
True and is a correct explanation |
|
B. True |
True but is not a correct explanation |
|
C. True |
Incorrect |
|
D. Incorrect |
Correct |
|
41. In the preparation of dry carbon dioxide, the gas is collected by upward displacement of air |
because |
Carbon dioxide does not mix with air |
|
42. Sodium chloride dissolves in water |
because |
Sodium chloride is formed by transfer of electrons from sodium to chlorine atoms |
|
43. Elements in group II of the Periodic Table are more reactive than those in group I |
because |
Group II elements need to lose two electrons in order to achieve stable noble gas structure |
|
44. Concentrated sulphuric acid is not used to dry ammonia |
because |
Ammonia is oxidized by concentrated sulphuric acid to nitrogen dioxide |
|
45. Chlorine is used to prepare anhydrous iron (II) chloride |
because |
Chlorine is an oxidizing agent |
In each of the questions 46 to 50 one or more of the answers given may be correct.Read each question carefully and then indicate the correct answer according to the following:
A. If 1, 2 and 3 only are correct
B. If 1 and 3 only are correct
C. If 2 and 4 only are correct
D. If 4 only is correct.
46. Which of the following is are true about electrolysis of dilute sulphuric acid?
1. Hydrogen is produced at the cathode
2. The acidity at the cathode increases
3. The volume of gas produced at the cathode is bigger than the one produced at the anode
4. The anode decreases in size
47. The full symbol of an elements is 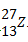 The ion of Z contains
The ion of Z contains
1. 10 neutrons
2. 10 electrons
3. 14 protons
4. 13 protons
48. Which one of the following is/are stages in sewage treatment?
1. Filtration
2. Chlorination
3. Use of aerobic bacteria
4. Addition of alum
49. Equal volumes of a 2M nitric acid and a 1M sulphuric acid
1. turn methyl orange indicator yellow
2. produce the same number of moles of hydrogen ions
3. produce equal volume of carbon dioxide when reacted with same amount of calcium carbonate
4. react with alkalis to form one mole of water
50. The graph below shows the variation in the volume of carbon dioxide evolved with time when hydrochloric acid was reacted with magnesium carbonate.
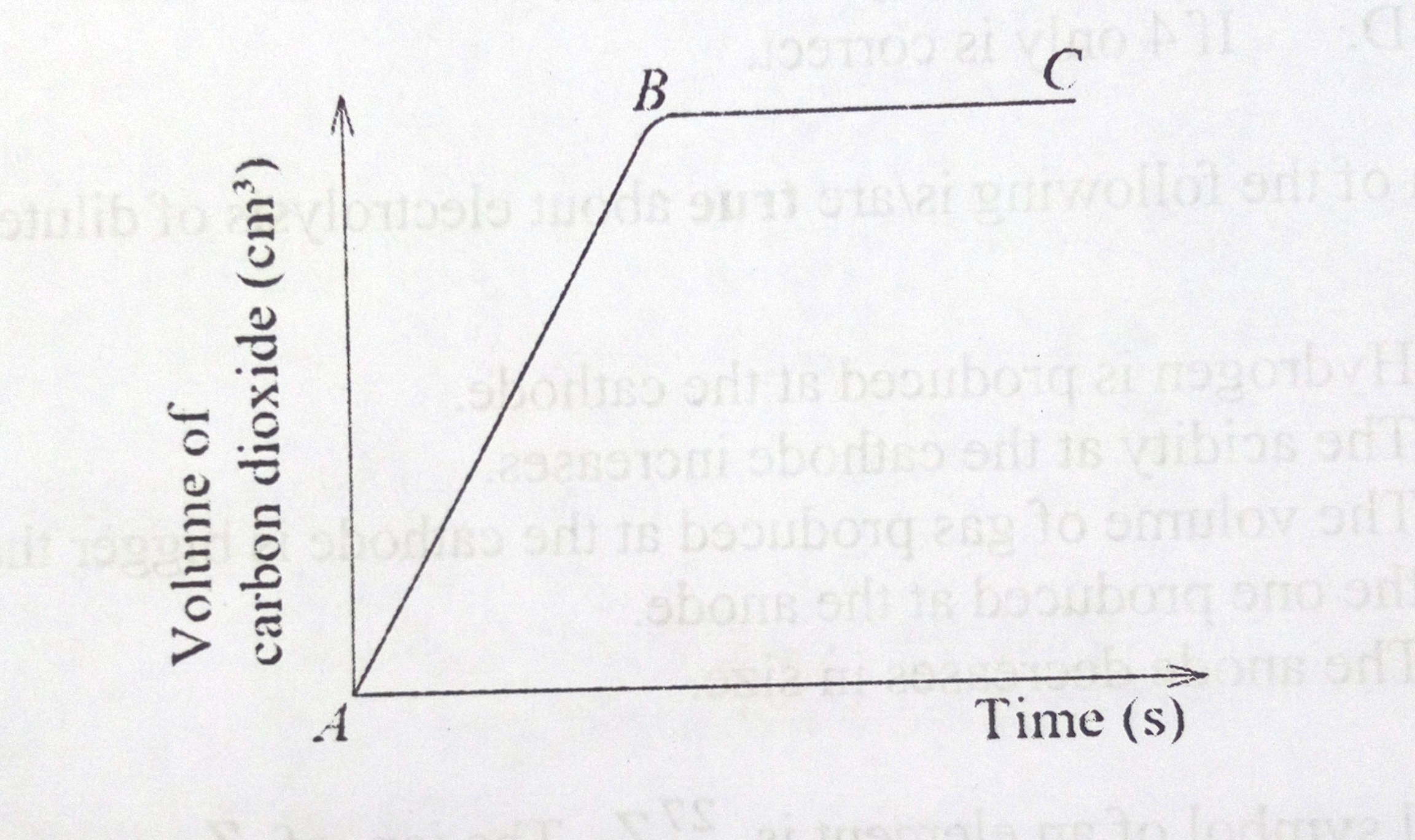
Which of the following conclusions can be made from the graph?
1. Reaction does not occur at the same rate throughout
2. Rate of evolution of carbon dioxide is greatest at the beginning
3. The portion B –C of the graph indicates the reaction is complete
4. The rate of the reaction increases with time
