1. Brass is an alloy of
A. Lead and tin
B. Iron and carbon
C. Copper and zinc
D. Magnesium and aluminum
2. A mixture of sodium carbonate and sodium hydrogen carbonate can best be separated by fractional crystallization because the two salts have different.
A. densities
B. solubilites
C. melting points
D. boiling points
3. The atomic number of an element T is 15. Which one of the following is the nature of the oxide of T?
A. Acidic
B. Neutral
C. Basic
D. Amphoteric
4. Which one of the following substances is formed when sodium is burnt in limited amount of air?
A. Sodium oxide
B. Sodium peroxide
C. Sodium carbonate
D. Sodium nitride
5. Which one of the following allotropes of sulphur is stable above 960C?
A. Monoclinic sulphur
B. Rhombic sulphur
C. Plastic sulphur
D. Amorphous sulphur.
6. The atomic numbers of elements W, X, Y and Z are 9, 11, 12, 14 respectively. Which one of the following pairs of elements can combine to form a covalent compound?
A. W and X
B. X and Y
C. Y and Z
D. Z and W
7. Which one of the following anions when in solution would form a yellow precipitate with lead (II) ions?
A. Cl-(aq)
B. CO32- (aq)
C. I-(aq)
D. SO42-(aq)
8. Which one of the following carbonates when heated decomposes without leaving a solid residue?
A. Ammonium carbonate.
B. Copper (II) carbonate
C. Magnesium carbonate
D. Lead (II) carbonate
9. In which one of the following gases will magnesium burn to form a white solid that will react with water to form ammonia?
A. N02
B. N20
C. NO
D. N2
10.The full symbol of an atom of an element Z is 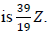 . Which one of the following is the number of neutrons in the nucleus of Z?
. Which one of the following is the number of neutrons in the nucleus of Z?
A. 19
B. 20
C. 39
D. 58
11. Which one of the following properties is true about carbon and sulphur?
Both elements
A. form covalent compounds only
B. form acidic oxides only
C. conduct electricity
D. have allotropes
12. Which one of the following substances can conduct electricity either in solution or molten state?
A. Hydrogen chloride
B. Sugar
C. Ethanol
D. Sulphur
Which one of the following substances contains the same number of moles as 10cm3 of a 0.5M nitric acid?
(1 mol of a gas occupies 22.4 dm3 at s.t.p; H = 1; C =12; N = 14)
A. 5.6dm3 of carbon dioxide at s.t.p
B. 17g of ammonia
C. 112cm3 of oxygen at s.t.p
D. 12g of carbon
14. Which one of the following metals is used in the laboratory preparation of hydrogen?
A. Iron
B. Zinc
C. Magnesium
D. Potassium
15. Which one of the following sets of compounds belongs to the same homologous series?
A. C2H4, C3H6 and C4H8
B. C2H6, C2H2 and C3H8
C. C2H2, C3H6 andC4H10
D. C2H6, C5H10 and C3H8
16. The atomic numbers of elements T,U,V and Z are 11,16,17 and 20 respectively. Which one of the elements forms an ion with a charge of negative two?
A. T
B. U
C. V
D. Z
17. Sulphuric acid reacts with sodium hydroxide according to the following equation.
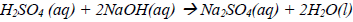
Which one of the following is the volume of a 2M sulphuric acid required to react completely with 10cm3 of a 2M sodium hydroxide solution?
A. 5.0 cm3
B. 10.0 cm3
C. 20.0 cm3
D. 40.0 cm3
18. When a piece of hot copper was lowered into a bell jar of air, the volume of air in the jar decrease. Which one of the following gases caused the decrease in the volume?
A. Water vapor
B. Carbon dioxide
C. Oxygen
D. Nitrogen
19. Which one of the following substances is not decomposed when strongly heated?
A. K2CO3
B. NaNO3
C. FeSO4
D. NaHCO3
20. Which one of the following pairs of substances will react when strongly heated together?
A. Magnesium oxide and iron
B. Zinc and aluminum oxide
C. Iron (III) oxide and copper
D. Lead (II) oxide and magnesium
21. Ammonia burns in oxygen according to the following equation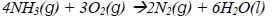
The maximum volume of oxygen required to burn 60cm3 of ammonia is
A. 45cm3
B. 80 cm3
C. 90 cm3
D. 180 cm3
22. The electronic configurations of elements X and Y?
A. XY3
B. X2Y3
C. X2Y
D. X3Y2
23. Which one of the following equations represents the reaction that does not take place during the manufacture of nitric acid from ammonia?

24. 0.4g of metal hydroxide MOH reacted completely with 20cm3 of a 0.5M hydrochloric acid. The relative formula mass of MOH is
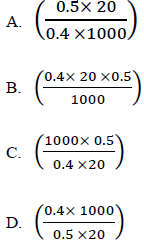
25. Which one of the following carbonates will react with dilute sulphuric acid to give a blue solution and a gas that turns lime water milky?
A. Zinc carbonate
B. Iron (II) carbonate
C. Magnesium carbonate
D. Copper (II) carbonate
26. Which one of the following substances will react with ammonium chloride to form ammonia?
A. HNO3
B. CuO
C. KOH
D. SO2
27. When a solution containing 2g of sodium hydroxide was completely reacted with hydrochloric acid, 2730J of heat was evolved. Which one of the following is the heat of neutralization of sodium hydroxide by hydrochloric acid? (NaOH = 40)
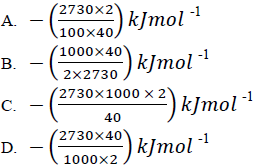
28. Which one of the following gases can bleach flowers but not litmus paper?
A. Sulphur dioxide
B. Nitrogen dioxide
C. Sulphur trioxide
D. Chlorine
29. Which one of the following is the concentration in grams per litre of a solution that contains 0.05 moles of sodium chloride in 50cm3? (NaCl =58.5).

30. Which one of the following statements is true about chlorine?
A. It displaces fluorine from solution of its salts
B. It is a reducing agent
C. It is less dense than air
D. It forms a precipitate with lead (II)nitrate solution
31. Which one of the following equations represents a redox reaction?
32. When 0.52g of methanol was burnt, the heat evolved raised the temperature of 85g of water from 20.30C to 53.30C. Which one of the following is the molar heat of combustion of methanol? (The specific heat capacity of water = 4.2Jg-1 K-1, C=12, H = 1, O=16)
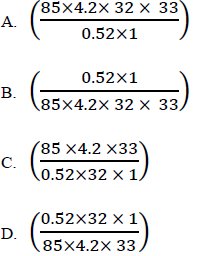
33. Which one of the following acids when reacted with a given mass of copper (II) carbonate will liberate the least amount of carbon dioxide?
A. 1M sulphuric acid
B. 2M nitric acid
C. 2M ethanoic acid
D. 2M hydrochloric acid
34. The mass of oxalic acid (H2C2O4) required to prepare 250cm3 of a 1.5M solution of the acid is
(H =1; C= 12; O= 16)
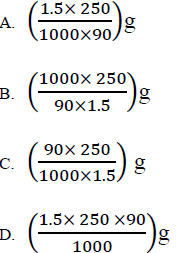
35. Which one of the following is/ are produced when a small amount of carbon dioxide is bubbled into sodium hydroxide solution.
A. Sodium hydrogen carbonate and water
B. Sodium carbonate and water
C. Sodium hydrogen carbonate only
D. Sodium carbonate only
36. The order of reactivity of the elements X,Y and Z is Z>X>Y. Which one of the following equations represents a possible reaction?
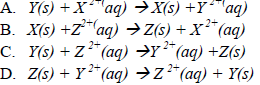
37. Chlorine reacts withiron from iron (III) chloride according to the following equation.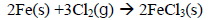
Which one of the following would be the volume of chlorine that would react with 5.6g of iron to produce (III) chloride at s.t.p?
(Fe = 56; 1 mole of a gas occupies 22.4 litres at s.t.p)
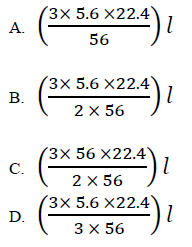
38. The formula of the ion formed when excess ammonia is added to aqueous solution of copper (II) ions is
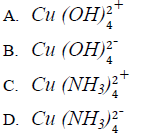
39. The electronic configuration of an atom of element G is 2:8:2. Which one of the following elements will show properties similar to G?
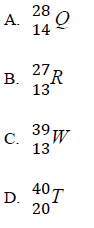
40. Ammonia reacts with copper(II) oxide to form copper according to the following equation.
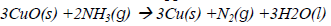
The mass of copper formed when 12g of ammonia is reacted with copper (II) oxide is
(Cu = 64; N = 16; H=1)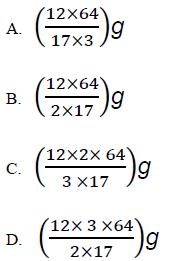
Each of the questions 41 to 45 consists of an assertion (statement) on the left-hand side and a reason on the right- hand side
Select
A. if both the assertion and reason are true statements and the reason is a correct explanation of the assertion
B. if both the assertion and reason are true statements but the reason is not a correct explanation of the assertion.
C. if the assertion is true but the reason is not a correct statement
D. if the assertion is not correct but the reason is a correct statement
INSTRUCTIONS SUMMARISED:
|
|
Assertion |
Reason |
|
A |
True |
True (Reason is a correct explanation) |
|
B |
True |
True (Reason is not a correct explanation) |
|
C |
True |
Incorrect |
|
D |
Incorrect |
Correct |
|
41. Water can be separated from its mixture with cooking oil by using a separating funnel
|
because |
Water and cooking oil have different boiling points |
|
42. In the manufacture of sulphuric acid by the contact process, Sulphur trioxide is dissolved in concentrated sulphuric acid instead of water
|
because |
Hard water contains sulphates and hydrogen carbonate ions |
|
43. Washing with hard water takes a lot of soap
|
because |
Hard water contains sulphates and hydrogen carbonate ions |
|
44.Concentrated nitric acid reacts with sulphur to form sulphur dioxide
|
because |
the concentrated acid is an oxidizing agent |
|
45.Powdered zinc reacts faster with hydrochloric acid than equal mass of zinc foil |
because |
Zinc powder contains more atoms than zinc foil |
46. Which of the following salts can be prepared by passing dry hydrogen chloride over the heated metal?
1. CuCl2
2. ZnCl2
3. FeCl3
4. MgCl2
47. Which of the following is/are characteristics of alkens? Alkenes
1. are hydrocarbons
2. decolourise bromine water
3. burn to form water and carbon dioxide
4. are saturated compounds
48. Which of the following oxides is/are soluble in excess potassium hydroxide solution?
1. PbO
2. CuO
3. ZnO
4. FeO
49. Which of the following is/ are property(ies) of aqueous hydrogen chloride?
1. It reacts with copper to form hydrogen
2. it reacts with carbonates to form carbon dioxide
3. It bleaches litmus paper
4. It reacts with calcium oxide to form a salt and water
50. Which of the following is/are true about the product obtained when copper is heated in air?
1. It is black solid
2. It reacts with sodium hydroxide
3. It reacts with nitric acid
4. It is a brown solid
