1. Which one of the following is a physical change?
A. Heating ammonium chloride
B. Heating potassium nitrate
C. Burning sulphur
D. Rusting of iron
2. Which one of the following carbonates does not decompose when heated?
A. (NH4)2CO3
B. K2CO3
C. CaCO3
D. FeCO3
3. Which one of the following gases does not burn?
A. Nitrogen
B. Hydrogen
C. Methane
D. Carbon monoxide
4. Which one of the following metals can react with steam?
A. Copper
B. Lead
C. Silver
D. Iron
5. Which one of the following is not a characteristic of a liquid? It has
A. a definite shape
B. a fixed volume
C. mobile molecules
D. weak forces between molecules
6. Which one of the following is the reaction which leads to the formation of soap?
A. Hydrogenation
B. Polymerisation
C. Saponification
D. Dehydration
7. Which one of the following is a use of chlorine?
A. Sewage treatment
B. Food preservation
C. Manufacture of plastics
D. Manufacture of fertilizers
8. Which one of the following is a strong electrolyte?
A. Citric acid
B. Ethanoic acid
C. Ammonia solution
D. Sodium hydroxide solution
9. The formula of the ion of an element X is X3+ If the electronic configuration of the ion is 2:8, to which group in the Periodic Table does X belong?
A. II
B. III
C. V
D. VIII
10. The process by which glucose can be converter=d into ethanol is called
A. fermentation
B. hydrogenation
C. dehydration
D. polymerization
11. Which one of the following elements will burn in oxygen to form acidic oxides?
A. Ca
B. Zn
C. K
D. S
12. Which one of the following forms of carbon is used to absorb brown color from crude sugar?
A. Wood charcoal
B. Sugar charcoal
C. Animal charcoal
D. Lamp charcoal
13. Which one of the following oxides will dissolve in sodium hydroxide solution?
A. PbO
B. CuO
C. CaO
D. FeO
14. Which one of the following substances can be used to distinguish ethene from ethane?
A. Bromine water
B. Lime water
C. Litmus paper
D. A glowing splint
15. Which one of the following is not a property of a base?
A. All bases react with acids to form water and slats
B. Solutions of all bases turn red litmus paper blue
C. All bases liberate ammonia from ammonium salts
D. Some bases are not soluble in water
16. The substances produced during electrolysis of brine using a mercury cathode cell are
A. sodium hydroxide, oxygen and chlorine
B. hydrogen, chlorine and sodium hydroxide
C. hydrogen, sodium and chlorine.
D. sodium, oxygen and hydrogen.
17. The atomic numbers of elements X and Y are 17 and 20 respectively. Which one of the following occurs when X combines with Y?
A. Each atom of X loses one electron
B. An atom of Y gains two electrons
C. The compound formed contains equal numbers of X and Y ions
D. The solution of the compound formed conducts electricity.
18. Which one of the following sulphates is prepared by precipitation method?
A. Iron(II)sulphate
B. Copper(II) sulphate
C. Barium sulphate
D. Zinc sulphate
19. Which one of the following hydrocarbons is unsaturated?
A. C4H10
B. C3H8
C. C2H6
D. C2H4
20. Which one of the following nitrates when strongly heated will decompose to form a metal?
A. Cu(NO3)2
B. Pb(NO3)2
C. AgNO3
D. NaNO3
21. Which one of the following anions will react with silver nitrate solution to form a white precipitate insoluble in dilute nitrate acid?
A. Chloride
B. Sulphite
C. Carbonate
D. Hydrogen carbonate
22. Which one of the following sets of substances is produced when ammonium carbonate is heated?
A. Ammonia, carbon monoxide, water.
B. Nitrogen, carbon dioxide, water
C. Ammonia, water, carbon dioxide
D. Hydrogen, nitrogen, carbon monoxide.
23. The order of reactivity of elements X, Z and hydrogen is X>H>Z. Which one of the following statements about the elements is true?
A. X liberates hydrogen gas from dilute hydrochloric acid.
B. Z liberates hydrogen gas from sulphuric acid.
C. Z displaces from a solution containing its ions
D. Hydrogen reduces the oxide of X.
24. The mass of 2 × 10 23 atoms of aluminum is
(the atomic mass of aluminium = 27, Avogadro’s Number = 6 ×1023 particles)
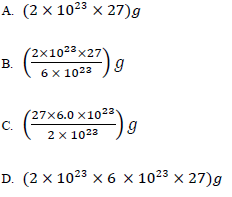
25. The table below shows number if neutrons in atoms W, X,Y and Z: and their atomic masses. Which of the atoms are isotopes?
|
Atom |
Atomic mass |
Number of neutrons |
|
W |
39 |
20 |
|
X |
40 |
20 |
|
Y |
40 |
22 |
|
Z |
41 |
22 |
W and X
X and Y
Y and Z
W and z
26. When a solution of barium nitrate that was acidified with dilute nitric acid was added to solution X, a white precipitate was formed The anion in X is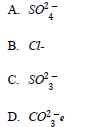
27. Which one of the following gases diffuses fastest? (C= 12, O = 16; N =14; Cl = 35.5; H = 1)
A. CO2
B. CH4
C. HCl
D. NO
28. Which one of the following cations will react with ammonia solution to form a precipitate which dissolves in excess ammonia solution?
Fe3+(aq)
Al3+(aq)
Pb2+(aq)
Zn2+(aq)
29. Iron reacts with oxygen to form iron(II) oxide according to the following Equation.
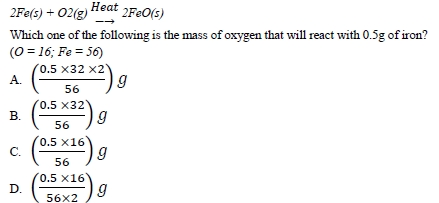
30. Propane burns in oxygen according to the following equation.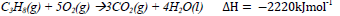
The volume of propane measured at room temperature that would be required to produce 4500kJ of heat on complete combustion is (1 mole of gas occupies 24.0dm3 at room temperature)
A. 59.20 cm3
B. 48.65 cm3
C. 11.84 dm3
D. 9.73dm3
31. Which one of the following mixtures when heated would form a residue which is yellow when hot? The mixture of Zinc and the oxide of
lead
aluminum
magnesium
calcium
Which one of the following is the mass of carbon dioxide that would occupy 5.6 cm3 at S.T.P?
(C= 12; O = 16; 1 mole of gas occupies 22.4 dm3)
A. 15.00g
B. 11.00g
C. 7.50g
D. 2.85g
34. Which one of the following compounds is used to prepare sulphur dioxide in the laboratory?
Na2S
Na2SO3
Na2SO4
NaHSO4
Lead(II) nitrate decomposes on heating according to the following equation.
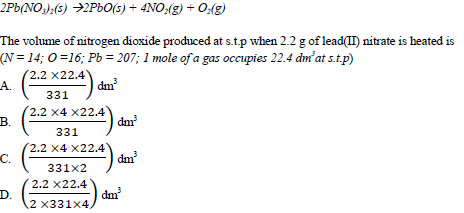
35. An aqueous solution, X, formed a white precipitate with barium nitrate solution. Which one of the following solutions will give the same observation as barium nitrate solution with X?
A. Ammonia solution
B. Lead(II) nitrate solution
C. Sodium sulphate solution
D. Zinc chloride solution
36. An atom of element Y has two energy levels. If Y forms an ion with the formula Y2-, what is the atomic number of Y?
A. 2
B. 4
C. 6
D. 8
37. The mass number of an atom is 11. if irs electronic configuration is 2:3, what is the number of neutrons in the atom?
A. 5
B. 6
C. 8
D. 11
38. 20cm3 of an acid HX required 25cm3 of 0.05M sodium carbonate solution for complete neutralization. The concentration of the acid in moles per liter is
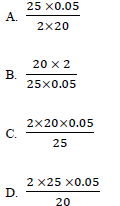
39. Solutions Q,R,T and X have pH values 14,10, 7 and 2 respectively. Which one of the solutions would react with copper (II) oxide to produce a salt and water?
A. Q
B. T
C. X
D. R
40. Hydrogen peroxide decomposes to give oxygen according to the following Equation.
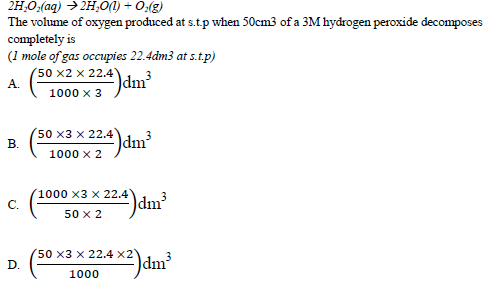
Each of the questions 41 to 45 consists of an assertion (statement) on the left – hand side and a reason on the right hand side.
Select
A. if both the assertion and reason are true statements and the reason is a correct explanation of the assertion.
B. if both the assertion and reason are true statements but the reason is not a correct explanation of the assertion.
C. if the assertion is true but the reason is not a correct statement
D. if the assertion is not correct but the reason us a correct statement.
INSTRUCTIONS SUMMARISED:
|
|
Assertion |
Reason |
|
A |
True |
True (Reason is a correct explanation) |
|
B |
True |
True (Reason is not a correct explanation) |
|
C |
True |
Incorrect |
|
D |
Incorrect |
Correct |
|
41. Copper (II) hydroxide dissolves in excess aqueous ammonia |
because |
Copper (II) hydroxide is amphoteric |
|
42. A mixture of ammonium chloride and sodium chloride can be separated by sublimation |
because |
they both have common anions |
|
43. Substance react faster at higher temperatures |
because |
Increase in temperature causes the reacting particles to be far |
|
44. The pH of carbon dioxide solution is less than seven |
because |
carbon dioxide is an acid |
|
45.When hydrogen chloride is bubbled into potassium iodide solution, a brown solution is formed |
because |
Chlorine is above iodine in the activity series. |
In each of the questions 46 to 50, one or more of the answers given may be correct. Read each question carefully and then indicate the correct answer according to the following.
A. If 1,2 and 3 only are correct
B. If 1 and 3 only are correct
C. If 2 and 4 only are correct
D. If 4 only is correct.
46. Which of the following is/ are true about the extraction of sodium by electrolysis?
1. The anode is made of iron
2. The cathode is made of carbon
3. The electrolyte is concentrated sodium chloride solution
4. The sodium is deposited at the cathode.
47. Which of the following substances is/ are formed when chlorine is bubbled into cold dilute potassium hydroxide solution?
1. H2O
2. KCl
3. KOCl
4. KClO3
48. Which of the following is/ are observed when sugar is warmed with concentrated sulphuric acid?
1. The sugar swells
2. Sugar turns black
3. Steam is evolved
4. Carbon monoxide is evolved.
49. Which of the following substances is/are formed when concentrated nitric acid reacts with sulphur?
1. Sulphuric acid
2. Nitrogen monoxide
3. Nitrogen dioxide
4. Sulphur dioxide
50. Which of the following is/are true about polythene? Polythene
1. is biodegradable
2. is a synthetic polymer
3. is a thermosetting plastic
4. can be recycled.
