SECTION A
Answer all questions in this section
1. a) State a method by which each of the following mixtures can be separated.
i) Iron (II) chloride and iron (II) oxide
ii) Sodium carbonate and sodium hydrogen carbonate.
b) Give a reason why it is possible to separate the mixture in (a) (i) using the method you have stated.
c) State what would be observed if iron (II) chloride solution was mixed with aqueous silver nitrate.
d) Write the formula of the residue formed when a mixture of sodium carbonate and sodium hydrogen carbonate is heated strongly.
2. The atomic numbers of elements X, Y and Z are 18, 16 and 19 respectively.
a) State the
i) group in the Periodic Table to which X belongs
ii) valency of Y
iii) period in the Periodic Table to which Z belongs.
b) Write the formula of the compound that can be formed when X reacts with Y and Z.
c) State one physical property of the compound formed between X and Y in which it differs from the compound formed between X and Z.
3. a) State the conditions under which oxygen can react with sulphur and copper.
b) Write equation for the reaction between oxygen and sulphur, oxygen and copper.
c) i) State which one of the compounds formed in (b) (i) and (ii) will react with dilute hydrochloric acid.
ii) Give a reason for your answer in (c) (i).
4. a) A mixture of iron filings and sulphur was heated strongly. Write equation for the reaction that took place.
b) Dilute sulphuric acid was added to the product in (a)
i) State what was observed
ii) Write equation for the reaction that took place
c) One of the substances formed in reaction (b) (ii) pollutes air.
5. Ammonia reacts with oxygen in the presence of hot platinum to produce a colorless gas X, which eventually gives brown fumes.
a) Identify X
b) Write equation to show the formation of
i) X
ii) the brown fumes.
c) State the
i) role of platinum
ii) industrial application of the reaction in (b)
6. A gaseous organic compound J contains 82.76% carbon, the rest being hydrogen.
a) To which group of organic compounds does J belongs?
b) Calculate the empirical formula of J
c) 140cm3 of J weighed 0.363g at s.t.p. Determine the molecular formula of J. (1 mole of a gas occupies 22400cm3 at s.t.p)
7. State what would be observed and write ionic equation for the reaction that would take place if hydrogen chloride was bubbled through aqueous.
a) sodium hydrogen carbonate.
i) Observation
ii) Equation
b) silver nitrate
i) Observation
ii) Equation
8. a) i) Name one process by which ethanol can be produced from sugar.
ii) Write equation for the production of ethanol by the process you have names in (a) (i).
b) Ethanol can be converted to ethane by dehydration.
i) State the conditions under which the reaction takes place.
ii) Write equation for the reaction leading to the formation of ethene from ethanol.
c) Write equation for the reaction between ethane and bromine.
9. a) State what is meant by the term enthalpy of combustion.
b) Carbon burns in oxygen according to the following equation.
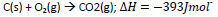
Calculate the
i) amount of heat evolved when 3.6g of carbon is burnt completely in oxygen. (C=12)
ii) volume of oxygen at s.t.p that would be required to produce 78.6kJ of heat. (1mole of gas occupies22.4dm3 at s.t.p)
10. Name one reagent that can be used to differentiate between the following pairs of ions, and in each case state what would be observed when each of the ions is treated separately with the reagent you have named.
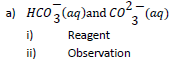

SECTION B
Answer any two questions from this section
11. a) Distinguish between the terms anode and cathode.
b) Explain why copper (II) chloride in solid form does not conduct electricity whereas in molten form it does.
c) A dilute solution off copper(II) chloride was electrolyzed using graphite as electrodes.
i) State what was observed at the cathode
ii) Write equation for the reactions at the anode and cathode respectively.
d) Describe how the product at the anode can be identified.
e) The electrolysis of dilute copper (II) chloride was repeated for sometime using copper instead of graphite as electrodes.
i) State what was observed at the anode and cathode respectively
ii) Write equation to support your observation at the anode.
f) State one factor other than change of electrodes from graphite to copper that would affect the products of electrolysis of copper(II) chloride solution and indicate how it would affect the process.
12. a) Describe how a dry sample of hydrogen can be prepared in the laboratory (Diagram not required).
b) Hydrogen burns in air o form liquid L.
i) Identify L
ii) Name a reagent that can be used to test for L and state what would be observed if L was treated with the reagent you have named.
c) Write equation to show the reaction of hydrogen with chlorine.
d) State the condition(s) under which hydrogen can react with copper(II)oxide and write equation for the reaction.
e) Hydrogen reacts with iron(II,III) oxide according to the following equation.
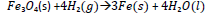
Calculate the volume of hydrogen measured at room temperature that would be required to produce 3.36g of iron.
(Fe = 56, 1 mole of gas occupies 24dm3 at room temperature)
g) State one industrial use of hydrogen.
13. a) Aluminum oxide is an amphoteric oxide.
i) Define the term amphoteric oxide
ii) Write equation to show the reaction of aluminum oxide with dilute nitric acid.
iii) Give two examples of amphoteric oxides other than aluminum oxide.
b) i) With the aid of an equation, describe how a pure dry sample of aluminum sulphate crystals can be prepared in the laboratory. Starting from aluminum oxide.
ii) Hydrated aluminum sulphate, Al2 (SO4)3.nH2O contains9.7% of aluminum. Calculate the value of n in the above formula. (Al = 27; S =32; O = 16; H =1)
c) i) Name one reagent that can be used to distinguish between aluminum ion and lead(II)ions.
ii) State what would be observed and write equation for the reaction that takes place if any, when the reagent you named in (c) (i) is treated separately with aluminum ions and lead(II)ions.
14. Haematite is one of the ores from which iron can be extracted.
a) Write the chemical formula of haematite
b) During the extraction of iron, roasted haematite is mixed with coke and limestone. The mixture is fed into the blast furnace and a blast of hot air blown into the furnace from the bottom.
i) Write equation(s) for the reaction(s) in the blast furnace that leads to the formation of iron.
ii) Explain the role of limestone
c) Write equation for the reaction of iron with
i) water
ii) hydrochloric acid.
d) To the resultant mixture in reaction (c) (ii) was added dilute ammonia solution until the alkali was in excess. State what was observed and write equation for the reaction that took place.
