1. Which one of the following substances will melt on heating strongly?
A. Iodine.
B. Sodium chloride.
C. Ammonium chloride.
D.Iron (III) chloride.
2.Which one of the following gases lights a growing splint?
A.Carbon dioxide.
B. Hydrogen.
C.Nitrogen.
D. Oxygen.
3.Which one of the following substances is used as a catalyst in the manufacture of nitric acid from ammonia?
A. Nickel
B.Iron.
C.Platinum.
D.Vanadium (V) oxide.
4.Which one of the following substances will form white fumes with hydrogen chloride?
A.Concentrated sodium hydroxide solution.
B.Concentrated nitric acid.
C.Concentrated sulphuric acid.
D.Concentrated ammonia solution.
5.Which one of the following substances when heated with ammonium chloride will produce ammonia?
A.NaOH.
B.CaSO4.
C.Pb(NO3)2.
D.MgCl2.
6.Which one of the following metals can be extracted from its ore by the reduction method?
A. Zn.
B.Al.
C.Ca.
D.Na.
7.Which one of the following gases is removed when air is passed through sodium hydroxide solution during the manufacture of oxygen?
A.Water vapour.
B.Carbon dioxide.
C.Rare gases.
D.Nitrogen.
8.The pH of solutions W, X, Y and Z are 3, 7, 10 and 14 respectively. Which one of the solutions will react with sodium carbonate to form carbon dioxide?
A.W.
B.X.
C.Y.
D. Z.
9.Which one of the following substances is not, chemically changed by passing an electric current through it?
A. Dilute sulphuric acid.
B. Molten candle wax.
C. Aqueous potassium sulphate.
D. Molten sodium chloride.
10. The atomic number of an element is 13. Which one of the following is the number of electrons in the outermost energy level of the particle?
A.2.
B. 3.
C. 8.
D. 10.
11.Which one of the following sodium salts when heated strongly will decompose to give oxygen?
A. Na2SO4.
B. NaNO2.
C. Na2SO3.
D. NaNO3.
12.Which one of the following is the percentage of the sodium carbonate in 28.2 g of hydrated sodium carbonate, Na2CO3.10H2O?
A. 9.86 %.
B. 26.20 %
C. 29.02%
D. 37.06%
13. Which one of the following carbonates is not suitable for the preparation of carbon dioxide using dilute sulphuric acd?
A. Sodium carbonate.
B. Zinc carbonate.
C. Lead (II) carbonate.
D. Copper (II) carbonate.
14. Which one of the following gases is evolved when copper is reacted with concentrated nitric acid?
A. Nitrogen.
B. Nitrogen monoxide.
C. Nitrogen dioxide.
D. Dinitrogen oxide.
15. The reaction between magnesium and dilute sulphuric acid to produce hydrogen shows the property of sulphuric acid as
A. An oxidizing agent.
B. A dehydrating agent.
C. A drying agent.
D. An acid.
16. Which one of the following substances is the bleaching agent in chlorine water?
A. HOCl.
B. HCl.
C. Cl2.
D. HClO3.
17. The graph below shows the variation in the volume of hydrogen evolved with time when excess zinc was reacted with dilute sulpuric acid using copper (II) sulphate as a catalyst.
Which one of the following is the best explanation for the shape of the graph between P and Q?
A. A layer of insoluble oxide formed on zinc sulphate.
B. The reaction became faster
C. Sulphuric acid was used up.
18. The catalyst was used up.The number of sulphate ions in 3.0 g of aluminum sulphate, Al2(SO4)3 is
(Al=27; S=32; O=16)
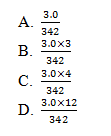
19.Which one of the following chlorides when in in solution will not form a precipitate when treated with sodium carbonate solution?
A. Magnesium chloride
B. Ammonium chloride.
C. Calcium chloride.
D. Zinc chloride.
20. Element M reacts with cold water while N reacts with steam. Q displaces M fro solution of its. Which one of the following is the order of reactivity of elements beginning with the most reactive?
A. Q, M, N.
B. M, N, Q.
C. N, M, Q
D. Q, N, M.
21. The atomic numbers of elements W, X, Y and Z are 3, 9, 11 and 19 respectively. Which one of the elements forms an ion with negative charge one?
A. W.
B. X.
C. Y.
D. Z.
22. Which one of following ions reacts with dilute hydrochloric acid to form sulphur dioxide?
A. Hydrogen sulphate ions.
B. Sulphate ions.
C. Sulphide ions.
D. Sulphite ions.
23. Which one of the following hydrocarbons when bubbled through bromine water will change the colour of solution from reddish – brown to colourless?
A. C2H4.
B. C3H8.
C. C2H6.
D. CH4.
24. Which one of the following sulphates when heated strongly will decompose to give sulphur dioxide and a reddish – brown solid?
A. CuSO4
B. (NH4)2SO4.
C. FeSO4.
D. Al2(SO)4.
25. 20 cm3 of 0.30 M sodium hydroxide was neutralised by the same volume of 0.15 M solution of an acid X. the basicity of the acid is
A.1.
B. 2.
C. 3.
D. 4.
26.Which one of the following oxides will dissolve in dilute nitric acid but not in dilute sodium hydroxide?
A. Lead(II) oxide
B. Zinc oxide.
C. Aluminum oxide.
D. Iron (III) oxide.
27. Which one of the following pairs of metal ions form precipitates that are soluble in aqueous ammonia solution?
- Pb2+(aq) and Zn2+(aq).
- Al3+(aq) and Cu2+(aq) .
- Zn2+(aq) and Cu2+(aq).
- Pb2+(aq) and Al3+(aq).
28. 20 cm3 0f an acid HX was neutralized by 25 cm3 of a 0.05 M sodium carbonate. Which one of the following is the molarity of the acid?

29. Which one of the following substances is formed when ethanol is dehydrated by concentrated sulphuric acid?
A. C2H4.
B. CH4.
C. CO.
D. C.
30. Carbon monoxide burns in air to produce carbon dioxide according to the following equation;
2CO(g) + O2(g) ————→ 2CO2(g) Δ = - 572 kJ
Which one of the following is the molar heat of combustion of carbon monoxide?
A.1144 kJ mol-1
B. 572 kJ mol-1
C.286 kJ mol-1
D. 143 kJ mol-1
31.The graph below shows the physical changes which took place when a solid P was heated strongly.
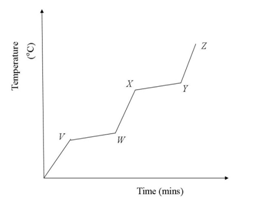
Which one of the following represents the boiling point of P?
A. V – W.
B. W – X.
C. X – Y.
D. Y – Z.
32.The atomic numbers of elements W, X, Y and Z are 12, 13, 15 and 19 respectively. Which one of the elements reacts readily with cold water?
A. W
B. X
C. Y
D. Z
33. Lead (II) ions react with iodide ions according to the following equation:
Pb2+(aq) + 2 I-(aq) ———→ PbI2(s)
Which one of the following is the volume of 1 M potassium iodide solution that would react completely with 20 cm3 of a 0.5 M lead (II) nitrate solution?
A. 5 cm3
B. 10 cm3
C. 20 cm3
D. 40 cm3
34. Which one of the following substances is formed during the electrolysis of concentrated sodium chloride using mercury cathode?
A. Sodium.
B. Oxygen.
C. Hydrogen.
D. Sodium hydroxide.
35. Which one of the following is the empirical formula of a hydrocarbon containing 88.88 % carbon? (H=1; C=12).
A. C4H6.
B. C2H3.
C. CH2.
D. CH
36. The atomic numbers of elements Y and Z are 6 and 17 respectively. Which one of the following is the formula of the compound formed between Y and Z?
A.YZ.
B. YZ2
C. YZ3
D. YZ4
37. Sodium hydrogen carbonate decomposes according to the equation when heated.
2 NaHCO3(s) ———→ Na2CO3(s) + CO2(g) + H2O(l)
The mass of sodium hydrogen carbonate which must be heated to give off 200 cm3 of carbon dioxide at room temperature is
(1 mole of gas at room temperature occupies 24,000 cm3, Na=23; O=16; C=12; H=1)
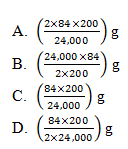
38. Which one of the following is observed when hydrogen is passed over heated lead (II) oxide?
A. A brown solid
B. A grey solid.
C. A black solid.
D. A yellow solid.
39. Which one of the following equations represents a reaction which is not carried out during the manufacture of sulphuric acid by the contact process?
A. S(s) + O2(g) ———→ SO2(g)
B. 2 SO2(g) + O2(g) ———→ 2 SO3(g)
C. SO3(g) + H2O(l) ———→ H2SO4(aq)
D. SO3(g) + H2SO4(aq) ——→ H2S2O7(l)
40. Hydrogen peroxide decomposes according to the following equation:
2 H2O2(l) ———→ 2 H2O(l) + O2(g)
Which one of the following is the volume of oxygen formed when 24.8 g of hydrogen peroxide is completely decomposed at s.t.p?
(H=1; O=16; One mole of a gas occupies 22.4 dm3 at s.t.p)
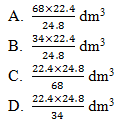
Each of the questions 41 to 45 consists of an assertion (statement) on the left – hand side and a reason on the right – hand side.
Select
A. If both the assertion and the reason are true statements and the reason is a correct explanation of the assertion.
B. If both the assertion and the reason are true statements but the reason is not a correct explanation of the assertion.
C. If the assertion is true but the reason is not a correct statement.
D. If the assertion is not correct but the reason is a correct statement.
INSTRUCTIONS SUMMARISED:
|
Assertion |
Reason |
|
True and is a correct explanation. |
41. A mixture of water and petrol can be separated using a separating funnel
because
water and petrol are immiscible liquids.
42. During the formation of sodium chloride, an electron is transferred from chloride to sodium
because
chlorine is a gas
43. Ethene undergoes polymerization reaction
because
ethene is a hydrocarbon.
44. The pH of a 2 M hydrochloric acid and that of a 2 M ethanoic acid are less than 7
because
both acid solutions contain the same concentration of hydrogen ions.
45. Aqueous solution of hydrogen chloride gas conducts electricity
Because
Hydrogen chloride gas is a covalent compound.
In each of the questions 46 to 50 one or more of the answers given may be correct. Read each question carefully and then indicate the correct answer according to the following.
A. If 1, 2 and 3 only are correct.
B. If 1 and 2 only are correct.
C. If 2 and 4 only are correct.
D. If 4 only is correct.
46. Which of the following is / are true about diamond and graphite?
1. Their atoms have the same mass number.
2. Both conduct electricity.
3. Both burns in excess air to produce carbon dioxide.
4. They both have layered structure.
47.Which of the following salts when dissolved in water cause(s) hardness in water?
1. Ammonium nitrate.
2. Calcium sulphate.
3. Potassium chloride.
4. Magnesium hydrogen carbonate.
48. Which of the following is / are true about ammonium hydroxide?
1. It turns methyl orange indicator yellow.
2. Its pH is less than 7.
3. It dissolves copper (II) hydroxide.
4. It turns the colour of phenolphthalein indicator to colourless.
49. The atomic numbers of elements Q and R are 6 and 16 respectively. Which of the following is / are the property / properties of the compound formed between Q and R?
1. It is very solute in water.
2. It has a high boiling point.
3. It conducts electricity.
4. It has a molecular structure.
50. Which of the following anions when in solution will rest with lead (II) nitrate solution to form a white precipitate?
1. CO32-
2. SO42-
3. Cl-
4. I-
END
