1a) i) What is menat by a conservative force?
ii) Give two examples of conservative force.
b) Explain the following
i) Damped oscillations
ii) Forced oscillations
c) i) State Newton’s law of gravitation.
ii) Show that Newton’s law of gravitation is consistent with Kepler’s third law.
d) If the earth takes 365 days to make one revolution around the sun, calculate the mass of the sun.
e) Explain briefly how satellites are used in world-wide radio or television communication
2. a) i) What is meant by fluid element and a flow line as applied to fluid flow?
ii) Explain why some fluids flow more easily than others.
b) i) State Bernoulli’s Principle
ii) Explain how a Pitot-static tube works
c) Air flowing over the upper surface of an air craft’s wings causes a lif force of 6.4 x 103 N. The air flows under the wings at a speed of 120ms-1 over an area of the upper surface of the air craft’s wings.
(Assume density of air = 1.2 kgm-3)
d) i) What is meant by surface tension and angle of contact of a liquid?
ii) A water drop of radius 0.5cm is broken up into other drops of water each of radius 1 mm. Assuming isothermal conditions, find the total work done to break up the water drop.
3. a) i) What is meant by efficiency of a machine?
ii) Describe an experiment to determine Young’s Modulus of a steel wire.
b) i) Define the impulse and momentum
ii) An engine pumps water such that the velocity of the water leaving the nozzle is 15ms-1. If there water jet is directed perpendicularly onto a wall and comes to a stop at the wall, calculate the pressure exerted on the wall.
c) i) Define inertia.
ii) Explain why a body placed on a rough plane will slide when the angle of inclination is increased.
d) i) State the conditions for a body to be in equilibrium under action of coplanar forces.
ii) Briefly explain the three states of equilibrium.
4. a) i) Define dimensions of a physical quantity.
ii) In the gas equation

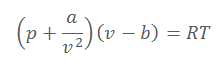
where p= pressure, V = volume, T = absolute temperature and R = gas constant, what are the dimensions of the constants a and b?
b) A particle is projected from a point on horizontal plane with a velocity, u at an angle, , above the horizontal. Show that the maximum horizontal range R max is given by
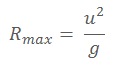
where, g, is acceleration due to gravity.
c) i) Define elastic limit of a material.
ii) Describe an experiment to determine Young’s Modulus of steel wire.
d) Explain why tyres of a vehicle travelling on a hard surfaced road may burst.
5.a) i) State Dalton’s law of partial pressures.
ii) Using the expression p = ⅓ρc, where p is the pressure of a gas of density ρ and mean square speed![]() derive Daltons law of partial pressures for two gases.
derive Daltons law of partial pressures for two gases.
b) i) What is meant by isothermal process and adiabatic process.
ii) Explain why adiabatic expansion of a gas causes cooling.
c) A gas at a temperature of 170C and pressure 1.0 x 10 5Pa is compressed isothermally to half its original volume. It is then allowed to expand adiabatically to its original volume.
i) Sketch on a P – V curve the above processes.
ii) If the specific heat capacity at constant pressure is 2100 Jmol-1 K-1 and at constant volume is 1500 Jmol -1 K -1, find the final temperature of the gas.
d) i) What is meant by a saturated vapour?
ii) Explain briefly the effect of altitude on the boiling point of a liquid.
6. a) i) Define specific latent heat of fusion.
ii) State the effect of impurities on melting point.
b) Explain why there is no change in temperature when a substance is melting.
c) With the aid of a labeled diagram, describe the continuous flow method of measuring the specific heat capacity of a liquid.
d) In an experiment to determine the specific latent heat of fusion of ice a heating coil is placed in a filter funnel and surrounded by lumps of ice. The following two sets of readings were obtained.
|
V(V) |
4.0 |
6.0 |
|
I (A) |
2.0 |
3.0 |
|
Mass of water m(g) collected in 500s. |
14.9 |
29.8 |
Calculate the;
i) specific latent heat of fusion of ice.
ii) energy gained in the course of obtaining the first set if readings.
e) Why are two sets of readings necessary in (d) above.
7. a) i) Define a black body.
ii) Sketch and explain graphs of intensity versus wavelength for three different temperatures of a black body.
b) Describe with the aid of a labeled diagram how an optical radiation pyrometer is used to measure temperature.
c) i) State Prevost’s theory of heat exchanges.
ii) A metal sphere of radius 1.5cm is suspended within an evacuated enclosure whose walls are at 320k. The emissivity of the metal is 0.40. Find the power input required to maintain the sphere at a temperature of 320K, if heat conduction along the supports is negligible.
d) A metal boiler is 1.5cm thick. Find the difference in temperature between the inner and outer surfaces if 40kg of water evaporate from the boiler per metre squared per hour.
(Latent heat of vaporization of water = 2268kJKg-1, Thermal conductivity of the metal of the boiler = 63Wm-1K-1).
8. a) i) Distinguish between mass defect and binding energy of an atomic nucleus.
ii) Sketch a graph of nuclear binding energy per nucleon versus mass number for naturally occurring isotopes and use it to distinguish between nuclear fission and fusion.
b) Describe with the aid of labeled diagram, Millikan’s oil drop experiment to determine charge on an oil drop.
c) i) Explain briefly diffraction of X-rays by crystals and derive Bragg’s law.
ii) A second order diffraction image is obtained by reflection of X-rays at atomic planes of a crystal for a glancing angle of 110 24’. Calculate the atomic spacing of the planes if the wavelength of X-rays is 4.0 x 10 -11 m.
9. a) State Bohr’s model of an atom.
b) An electron of mass m and charge –e, is considered to move in circular orbit about a proton.
i) Write down the expression for the electric force on the electron.
ii) Derive an expression for the total energy of the electron given that the angular momentum of the electron is equal to![]() where n is an integer and h is Planck’s constant.
where n is an integer and h is Planck’s constant.
c) With the aid of a labeled diagram, describe the operation of a diffusion type cloud chamber.
d) The energy levels of an atom have values
E1 = 21.4 eV
E2 = -4.87 eV
E3 = - 2.77 eV
E4 = -0.81 eV
E = 0.00 eV
i) Calculate the wavelength of radiation emitted when an electron makes a transition from E3 and E2.
ii) State the region of the electromagnetic spectrum where the radiation lies.
10. a) Describe how positive rays are produced.
b) Describe how a Bainbridge spectrometer can be used to detect isotopes.
c) i) What is a time base as applied to a Cathode Ray Oscilloscope?
ii) Draw a sketch graph showing the variation of time-base voltage with time.
d) An alternating p.d to the Y-plates of an oscilloscope produces five complete waves on a 10cm length of the screen when the time base setting is 10mscm-1. Find the frequency of the alternating voltage.
e) i) Explain the motion of an electron projected perpendicularly into a uniform magnetic field.
ii) An electron accelerated from res
t by a p.d of 100V, enters perpendicularly into a uniform electric field of intensity 105Vm-1. Find the magnetic flux density, B which must be applied perpendicularly to the electric field so that the electron passes undeflected through the fields.
END
