PAPER 1
SECTION A
Answer all questions in this section
1. Complete the following equations
![]()
![]()
![]()
![]()
2. (a) (i) What is meant by the term ionization energy of an element.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii) Write equation to show the first ionization of magnesium
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b) The second and third ionization energies of magnesium are 1450 kj mol-1 and 7730 kj mol-1 respectively
Give a reason for the large difference between the second and the third ionization energies of magnesium
……………………………………………………………………………………………………………………………………………………………………………………………………..
3. The second dissociation constant of a dibasic acid is 4.39x10-5 mol2 dm-6 at 250C
(a) Calculate the degree of 0.01M solution of the acid
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)Sketch a diagram to show how the degree of dissociation varies with dilution
4. (a) (i)Write the electronic configuration of aluminium.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii) State the type of bonding that exists in aluminium fluoride
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b) By using equations only, explain why aqueous solution of aluminium chloride is acidic
……………………………………………………………………………………………………………………………………………………………………………………………………..
5. (a)Draw and name the structure of the compounds.
Structure Name
(i) NCl3. <
(ii) PCl5
(b)Write equation for the reaction between nitrogen trichloride and boron trichloride
……………………………………………………………………………………………………………………………………………………………………………………………………..
6. Name one reagent that can be used to distinguish between each of the following pairs of compounds and in each case state what would be observed if each member of the pair is treated with the reagent.
(a) CH3CH2CHCH3 and CH3CH2CH2OH
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b) (CH3CH2)2NH and CH3CH2NH2
……………………………………………………………………………………………………………………………………………………………………………………………………..
7. Ethanol decomposes according to the equation
CH2CHO (g) ⇄ CH4(g) + CO(g) ∆H=-ve
(a)Write an expression for the equilibrium constant kp for the reaction
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)Explain how kp would be affected if
(i) the temperature is increased
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii) pressure is increased
……………………………………………………………………………………………………………………………………………………………………………………………………..
8. Write equation to show how benzene can be converted to
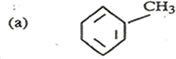
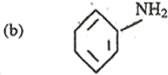
……………………………………………………………………………………………………………………………………………………………………………………………………..
9. 1.00 dm3 of aqueous solution contains 5.00g of butanoic acid. Calculate the mass of butanoic extracted with the solution was shaken.
(a) with50cm3 of a solvent Q
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b) twice with 25cm3 of solvent Q
……………………………………………………………………………………………………………………………………………………………………………………………………..
SECTION B
Answer six questions in this section
10.(a)Name two types of polymerisation reactions.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)State the structural requirements for the formation of polymers by each of the two reactions you have named in (a)
……………………………………………………………………………………………………………………………………………………………………………………………………..
(c) Write names and structural formulae of
(i) two naturally occurring polymers
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii)the monomers of polymers named in c(i)above
……………………………………………………………………………………………………………………………………………………………………………………………………..
11. (a) Write the formula for an ore of aluminium.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)During the extraction of aluminium the ore is first treated with sodium hydroxide followed by aluminium hydroxide
(i)State the purpose of adding sodium hydroxide
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii)write equation for the reaction between the ore and sodium hydroxide
……………………………………………………………………………………………………………………………………………………………………………………………………..
(iii)what is the purpose of adding aluminium hydroxide
……………………………………………………………………………………………………………………………………………………………………………………………………..
(c) Briefly explain how aluminium can be obtained after the ore has been treated as in (b)
……………………………………………………………………………………………………………………………………………………………………………………………………..
(d) Carbon dioxide was used instead of aluminium hydroxide in (b)write equation for the reaction that took place
……………………………………………………………………………………………………………………………………………………………………………………………………..
12. (a) write equation for the solubility of silver bromide in water at 250C
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b) If the solubility product of silver bromide at 250C is 5.0x10-13 mol2 dm-6
Calculate the solubility in g dm-3 at 250C of silver bromide in
(i)water
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii) 0.1M hydrobromic acid. (state any assumptions made)
……………………………………………………………………………………………………………………………………………………………………………………………………..
(c)State two methods that can be used to determine solubility product
……………………………………………………………………………………………………………………………………………………………………………………………………..
13. The graph shows the distribution of ammonia between layer of 0.1M copper(II) ions and chloroform
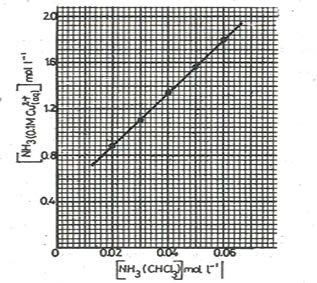
(a) (i)Determine the distribution coefficient KD of ammonia between aqueous copper(II) ions and chloroform.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii)State what the value of KD you have determined indicates about the distribution of ammonia
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)The graph does not pass through the origin because ammonia reacts with copper(II) ions
(i)Determine the number of moles of ammonia that reacts with copper(II) ions
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii)Write equation for the reaction between ammonia and copper(II) ions
……………………………………………………………………………………………………………………………………………………………………………………………………..
14. (a)Write the structural formulae and names of all possible isomers of an organic compound having a molecular formula C3H8O.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)When one of the isomers P in (a) above was reacted with acidified potassium dichromate compound Q was formed. Q reacted with phosphorus pentachloride to form compound R and hydrogen chloride gas
Identify
P……………………………………………………………………………………………..
Q………………………………………………………………………………………………
R…………………………………………………………………………………………..
(c)Write equation and indicate a mechanism for the reaction between P and concentrated sulphuric acid
……………………………………………………………………………………………………………………………………………………………………………………………………
15. Name a reagent that can be use to distinguish each of the following pairs of compounds. State what would be observed if the compounds are treated with the regeant:

…………………………………………………………………………………………………………………………………………………………………………………………………..

……………………………………………………………………………………………………………………………………………………………………………………..
(c) CH3CH2CH=CH2 and CH3CH2C≡CH
……………………………………………………………………………………………………………………………………………………………………………………………………..
16. (a) State three characteristics of chromium as a transition metal.
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b) A solution of potassium dichromate (VI) was added to an acidified solution of iron(II)sulphate
(i) state what was observed
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii) Write the ionic equation for the reaction that took place
……………………………………………………………………………………………………………………………………………………………………………………………………..
(c) (i)Write the structural formulae of chromium (III)chloride CrCl3.6H2O
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii)State one way of distinguishing the isomers
……………………………………………………………………………………………………………………………………………………………………………………………………..
17. When 0.i55g of an organic compound T was burned in oxygen 0.220g of carbon dioxide and 0.135g of water were formed
(a) Determine the empirical formula of T
……………………………………………………………………………………………………………………………………………………………………………………………………..
(b)when 0.225g of T at 1270C and 760mm Hg it occupied a volume of 119.11 cm3.(Molar volume of gas at s.t.p is 22.4 dm3)
(i)Calculate the molecular mass of T
……………………………………………………………………………………………………………………………………………………………………………………………………..
(ii)Determine the molecular formula of T
……………………………………………………………………………………………………………………………………………………………………………………………………..
(c)T reacts with acidified potassium dichromate to form ethane1,2-dioc acid
Write the formula and the IUPAC name of T
……………………………………………………………………………………………………………………………………………………………………………………………………..

PAPER 2
SECTIONA
Answer three questions from this section
1. Explain each of the following observations.
(a) dimethylamine is a stronger base than phenylamine
(b) the first ionization energy of aluminium is less than that of magnesium.
(c) the pH of a solution of chromium(III) sulphate is less than 7.
(d)1-bromohexane undergoes nucleophilic substitution reaction whereas bromobenzene does not.
(e) when solid lead(IV) chloride is added to water while fumes are observed and a brown precipitate is formed .
2. (a) Describe the spectrum of hydrogen atom.
Use a diagram to illustrate your answer.
(b)Explain how the spectrum of a hydrogen atom.
(i) is formed
(ii) provides evidence for the existence of energy levels in atoms.
(c) The frequency of hydrogen at a point of ionization is 32.8x1014 Hz
Calculate the ionization energy of hydrogen
(planks constant=6.6x10-34 js)
3.(a)Write equation to show how the following compounds can be prepared
(i) phenylamine(aniline)
(ii) amino ethane(ethylamine)
(b) which one of phenylamine and ethylamine is a stronger base? Explain your answer.
(c) write equations for each of the compounds phenylamine and ethylamine reacting with
(i) ethanoyl chloride (CH3COCl)
(ii) acidified solutions of sodium nitrite at 50C.
(d) (i) Write a mechanism for the reaction of ethanoyl chloride with ethylamine
(ii)How can the reaction in c(ii) above to be used to distinguish between phenylamine and ethylamine
(e)Phenylamine can be converted to benzene diazonium chloride
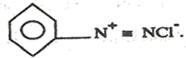
Write equations (reagents and conditions to be given) for the conversion of diozonium salt into
(i) iodo benzene
(ii) benzoic acid
4. (a) The melting point of 4-nitrophenol is much higher than that of 2-nitrphenol the two compounds can be separated by steam distillation.
(i) Explain why the melting point of 4-nitrphenol is higher than that of 2-nitrophenol.
(ii) Explain the principles of steam distillation.
(iii) Describe how a mixture of 2-nitrophenol and 4-nitrophenol can be separated by steam distillation.
(b) When a substance Y was distilled at 930C and 750mm Hg the distillate contained 55% of Y by mass. Calculate the relative molecular mass of Y.(The partial vapour pressure of water at 930C is 654mm Hg).
SECTION B
Answer two questions from this section.
5. The atomic numbers and melting points of some elements in period 3 of the periodic table are shown below
|
Element |
Na |
Mg |
Al |
Si |
P |
|
Atomic number |
11 |
12 |
13 |
14 |
15 |
|
Melting point(0C) |
98 |
650 |
660 |
1410 |
44 |
(a) (i) Plot a graph of melting points against atomic numbers.
(ii)Explain the shape of the graph in (i).
(b)Describe and explain how oxides of magnesium, aluminium and silicon react with
(i)sodium hydroxide
(ii)hydrochloric acid
(c) State the type of bonding in the oxides of sodium and phosphorus.
6. Write equations to show how each of the following conversions can be affected and indicate the reagents and the conditions for the reactions
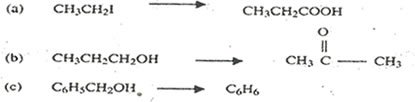
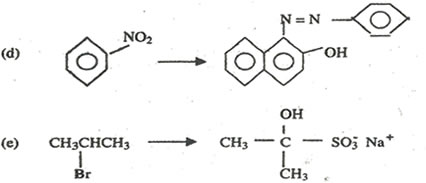
7. (a) Explain what is meant by the term ore.
(b) Describe two ways in which ores can be concentrated by
(i) physical method
(ii) chemical method
(illustrate your answer with suitable examples).
(c) A copper ore was dissolved in excess concentrated ammonia and the solution made up to1 dm3.the resultant solution was shaken with trichloromethane and left to settle.50cm3 of the organic layer needed 25.0cm3 of 0.05M hydrochloric acid for neutralisation.25cm3 of the aqueous layer was neutralised by 40cm3 of 0.5M hydrochloric acid.
Calculate the concentration of copper(II) ions in moles dm-3.
(the distribution coefficient of ammonia between water and trichloromethane is 25).
8. (a)Explain what is meant by the term melting point.
(b)State the factors which affect the melting point of
(i)metals
(i)molecular substances.
(c) Explain the trend in the melting points of the elements in group(II) and group(VII) of the periodic table.
(d) Explain why the transition metals of period 4 tend to have higher melting points than non-transitional metals of the same period.
(e) The table below shows the melting points of some compounds.
|
Compound Aluminium oxide Aluminium chloride Calcium oxide Calcium chloride |
Melting point/k 2290 451 2850 1051 |
Explain why:
(i)the melting point of aluminium chloride is abnormally low compared to that of aluminium oxide.
(ii)the melting point of calcium oxide is much higher than that of calcium chloride.
(f)Determine the freezing point depression for a solution containing 0.025g of sodium chloride in200g of water.(molar freezing point constant of water, kfp=1.860C mol1 kg-1,Na=23,Cl=35.5)
