PAPER 1
SECTION A
Answer all questions in this section
1. Write an ionic for the reaction between sodium hydroxide and
a) Silicon (iv)oxide, …………………………………………………………………………………………………………….
b) Lead(ii)oxide, ……………………………………………………………………………………………………………..
c) Aluminium(iii)oxide, ……………………………………………………………………………………………………………….
2. The mass spectrum of chlorine shaows peaks at masses 70, 72, and 74. The heights of the peaks respectively are in the ratio of 9:6:1
Calculate:
a) The average atomic mass of chlorine ………………………………………………………………………………………………………………………….
b) The relative abundance of 35Cl and 37Cl. ………………………………………………………………………………………………………………………
3. Complete the following equations and in each case write a mechanism for the reaction.
4. The table below shows the ionization energies (in k mol-1) of five elements lettered A, B, C, D, and E.
|
Element |
1st ionization energy |
2nd ionization energy
|
3rd ionization energy |
4th ionization energy |
|
A B C D E |
500 740 630 900 580
|
4600 1500 1600 1800 1800 |
6900 7700 3000 14800 2700
|
9500 10500 4800 21000 11600 |
a) Which one of these elements is more likely to form an ion with a charge of + 1? Give reason for your answer. ……………………………………………………………………………………………………………………………
b) State:
i. Two elements which belong to the same group in the periodic table. ………………………………………………………………………………………………………
ii. The group to which the elements you have stated in b(i) belong. ……………………………………………………………………………………………………………….
C) i) Write the formula of the chloride of element E. ……………………………………………………………………………………………………………………………………………………
ii) Write equation for the reactions between the chloride of element E and water. …………………………………………………………………………………………………………………………………………………….
5. a) Derive the expression for the half-life for a first order reaction:
2.303 log ? ; where ao is the initial concentration of the reactant and (ao-x) is the concentration at time, t. ……………………………………………………………………………………………………………………………..
b) The half-life of a first order reaction is 100s.
i. Calculate the rate constant. …………………………………………………………………………………………………………..
ii. Determine the percentage of the reactant that reacted after 250s. ……………………………………………………………………………………………………………….
6. Show how the following conversions could be carried out. ?
7. a) One of the properties of transition metals is complex ion formation
i. Define the term ‘complex ion’
ii. Explain why transition metals form many complexes.
c) Fe(CN)63- and (CuCl4)2- are complexes formed by iron and copper respectively.
State:
(i) The oxidation state of:
Iron …………………………………………………...........................
Copper ………………………………………………………………………..
(ii) The co-ordination number of:
Iron ……………………………………………………………………………
Copper ………………………………………………………………………….
8. (a) Define the term ‘partial pressure’.
(b) The vapour pressures of pure chloroform and carbon tetrachloride are 199.1 and 114.5mmHg respectively at 25oC. (Assume that a mixture of the two liquids behave as an ideal gas and that it contains 0.96 mole of each pure liquid.)
Calculate:
(i) The partial pressure of each component in the mixture. ................................................................................................................................................................................
(ii) The total pressure. ..............................................................................................................................................................................................
(c) Calculate the percentage of carbon tetrachloride in the vapour in equilibrium with the liquid mixture. ...................................................................................................................................................................................................
9. Complete the following equations and write the IUPAC name of the major organic product. ?
SECTION B
Answer only six questions from this section
10. State what would be observed and write equations for the reactions that take place when the following compounds are reacted.
a) Aqueous potassium dichromate (VI) with hydrogen sulphide. ……………………………………………………………………………………………………………………..
b) Aqueous iron(III) chloride with sodium carbonate. ………………………………………………………………………………………………………………………
c) Aqueous copper(II) sulphate with potassium iodide. ……………………………………………………………………………………………………………………….
11. (a) An organic compound A contains carbon, hydrogen and oxygen only. On combustion, 0.463g of A gave 1.1g carbon dioxide and 0.563g of water. Determine the empirical formula of A. ………………………………………………………………………………………………………………………………
(b) When vaporized, 0.1g of A occupies 54.5cm3 at 2080C and 98.3 KpA. Determine the molecular formula of A. …………………………………………………………………………………………………………………………………..
(c) A reacts with sodium metal with evolution of a gas. Write the structural formulae of all possible isomers of A. ………………………………………………………………………………………………………………………………
(d) A reacts with anhydrous Zinc chloride and concentrated hydrochloric acid to give a cloudy solution in about 5 minutes.
(i) Identify A. ………………………………………………………………………………………………………..
(ii) Show how A could be synthesized from but-2-nE. ……………………………………………………………………………………………………………………………..
12. a) A piece of clean magnesium ribbon was added to a solution of iron(III) chloride solution.
i. State what was observed. …………………………………………………………………………………………………………………….
ii. Explain your observation in (a) (i) above. …………………………………………………………………………………………………………………
iii. Write stepwise equations for the reactions that took place. …………………………………………………………………………………………………………………….
b) State what would be observed if a few drops of iron(III) chloride was added to the solution of the following:
(i) Sodium acetate.
(ii) Phenol
13. Complete the table below about the properties of different types of crystals.
|
Type of crystal |
Force holding the crystals |
Melting point (state whether low, moderate, high or very high) |
Form in which electricity is conducted if any. |
|
Metallic |
|
|
|
|
Ionic |
|
|
|
|
Network covalent |
|
|
|
14. a) Write equation to show how ethanol can be formed from glucose. ………………………………………………………………………………………………………………………………..
c) Write equations to show how ethyne can be:
i. Prepard from ethanol ……………………………………………………………………………………………………………
ii. Converted to methylpropyne ………………………………………………………………………………………………………………
d) i) Name one reagent that can be used to confirm the formation of methylpropyne. ………………………………………………………………………………………………………………………….
ii) State what would be observed if methylpropane was reacted with the reagent you have named in (c) (i) and write equation for the reaction. …………………………………………………………………………………………………………………………
15. a) The table below shows the atomic numbers of some elements and their electrons affinities.
|
Atomic no. |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
|
Electron affinity (kj mol-l) |
2.0 |
-6.7 |
3.0 |
13.5 |
6.0 |
20.0 |
36.4 |
i) Draw a graph of electron affinity versus atomic number.
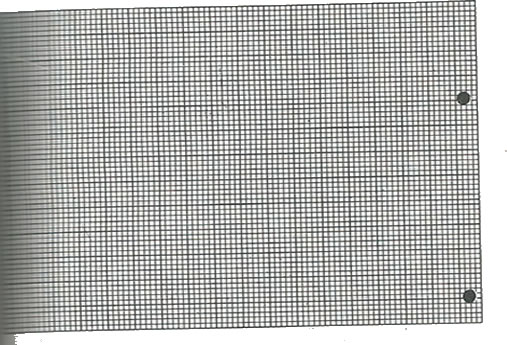
ii) Explain the shape of the graph.
b) List three factors which affect the size of the first ionization energy of an element.
16. Write equations to show how the following compounds can be synthesized.
(a) CH3CHO (from 1, 2- dibromoethane). …………………………………………………………………………………………………………
(b) CH3CO2H (from iodomethane). ………………………………………………………………………………………………………….
(c) CH3CO2CH3 (from ethane) …………………………………………………………………………………………………………
17. (a) Name two plants from which vegetable oil can be obtained. ……………………………………………………………………………………………………………………
(b) Soap was prepared from 9.5g of an oil containing mainly hexadecanoic acid CH3(CH2)14CO2H as the main component of the oil.
(i) Explain briefly how pure soap was obtained from the oil. ……………………………………………………………………………………………………………..
(ii) Write equation for the reaction leading to the formation of the soap. ………………………………………………………………………………………………………………..
(iii) Calculate the mass of the soap formed. …………………………………………………………………………………………………………………….
(d) Name one use of the residue left the oil has been extracted. …………………………………………………………………………………………………………………………..