1. Name a reagent that can be used to distinguish between the following pairs of compounds.
In each case, state what would be observed if each member of the pair was treated with the reagent you have named.
a) 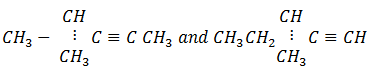
Reagent
Observation
b) Image
Reagent
Observation
2. Various concentrations of X and Y were reacted at a constant temperature. The table below shows the initial concentrations of X and Y and their initial rates for the reaction.
|
Experiment |
[X] (mol dm-3) |
[Y] (mol dm-3) |
Initial rate (mol s-1) |
|
1 |
0.2 |
0.2 |
3.5 × 10-4 |
|
2 |
0.4 |
0.4 |
1.4 × 10-3 |
|
3 |
0.8 |
0.4 |
5.6 × 10-3 |
a) State the order of reaction with respect to X and Y
i) X
ii) Y
b) Give reasons for your answers in (a)
c) Determine the overall order of the reaction
d) Calculate the value for the rate constant for the reaction
3.a) A solid Q contains 9.37% by mass of magnesium, 10.39% nitrogen and 42.18% water.
i) Calculate the empirical formula of Q
ii) Determine the molecular formula of Q (RFM of Q = 256 )
b) Solution of Q reacts with iron(II)sulphate in the presence oconcentrated sulphuric acid to form a brown ring. Identify Q.
c) Write equation for the reaction that would take place if Q was heated.
4. Complete the following equations and in each case, write a mechanism for the reaction
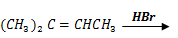
Mechanism:
b)
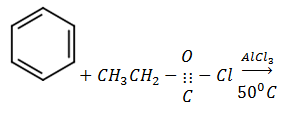
Mechanism:
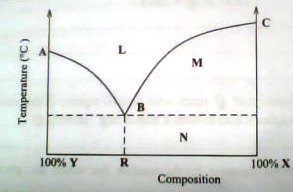
5. The temperature – composition diagram for a system containing two components X and Y is shown below
a) State what the following represents;
i) Regions:
L
M
N
ii) Points
A
B
C
iii) Curves
AB
BC
b) State what would happen when a mixture of composition R is heated.
6. Write equation for the reaction between aqueous sodium hydroxide and
a) chromium(III)oxide
b) beryllium oxide
c) tin(II)oxide
7.When a current of 0.65 A was passed through copper(II)sulphate solution using platinum electrodes for 35minutes, 0.0143g of hydrogen and 0.113g of oxygen were evolved.
a) Write equation for the reaction that took place at the
i) anode
ii) cathode
b) Determine the quantity of electricity required to evolve 1 mole gas at each electrode
i) At the anode
ii) At the cathode
8. State what would be observed and write equation for the reaction that would take place when:
a) excess concentrated hydrochloric acid was added to lead(II)oxide
b) potassium iodide was added to copper(II)sulphate solution
9. Write equation in each case to show how the following conversions can be effected:
a)
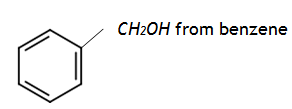
b) CH3 CONH2 from chloroethane
SECTION B
10. a) Silver chloride dissolves in warer according to the following equation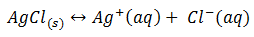
Write the expression for the solubility product, Ksp of silver chloride
b) The electrolytic conductivity of a saturated solution of silver chloride in water ar 250C is 3.41× 10-6 Ω-1 cm-1 and that of pure water is 1.6 ×10 -6 Ω-1 cm-1. Calculate the solubility product of a saturated solution of silver chloride at 250C.
(The molar conductivities at infinite dilution of silver nitrate, potassium nitrate and potassium chloride are 133.4, 145.0 and 149.9 Ω-1 cm2 mol-1 respectively at 250C)
c) Ammonia solution was added to a solution containing silver chloride
i) State how the solubility of silver chloride was affected
ii) Explain your answer in (c) (i) above.
11. Compound T, C3H6O reacts with 2,4- dinitrophenylhydrazine to form a yellow precipitate
a) Write the names and the structural formulae of all possible isomers of T
b) T reacts with ammoniacal silver nitrate solution to form silver
Identify T
c) Write equation and indicate a mechanism for the reaction between T and 2, 4 – dinitrophenylhydrazine under acidic condition
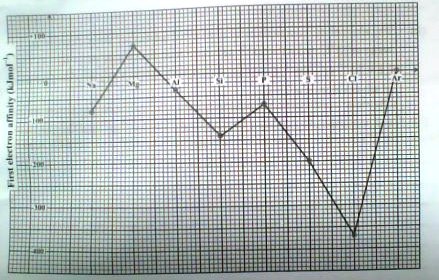
12. The figure below shows the variation of the first electron affinity of the elements in Period 3 of the Periodic Table
Explain each of the following observations:
i) There is a general increase in the first electron affinity from sodium to argon
ii) The first electron affinity of magnesium is higher than that of aluminum
iii) The first electron affinity of phosphorous is less than that of sulphur
13. Maganese is a d-block element in the Periodic Table
a) Define the term d-block element
b) i) Write the electronic configuration of manganese
ii) State the common oxidation states exhibited by manganese in its compounds
iii) Write the formulae of oxides of manganese each of the oxidation states you have stated in (b) (ii)
c) A black oxide, Y of manganese was fused with a mixture of potassium hydroxide and potassium nitrate to give a compound which when treated with water gave a green solution.
The green solution turned purple when acidified with sulphuric acid.
Identify:
i) Y
ii) the ion that gives the green solution its color
iii) the ion that gives the purple solution its color
d) Write ionic equation for the reaction leading to the formation of the purple solution
14 a) i) Sketch a greaph to show the pH change when hydrochloric acid is titrated with ammonia solution
ii) Explain the shape of your sketch graph in (a) (i)
b) Calculate the pH of a resultant solution formed when 10cm3 of a 0.1M sodium hydroxide solution is added to 25cm3 of a 0.1M Ethanoic acid at 250C
(Dissociation constant of Ethanoic acid at 250C = 1.8 × 10-5 moldm-3)
15. a) Beryllium. magnesium, calcium and barium are some of the elements that belong to Group II of the Periodic Table.
State how the elements react with sulphuric acid and give the conditions for the reactions
b) i) State how the solubilities of the sulphates of Group II elements vary down the group.
ii) Explain your answer in (b) (i)
c) Write equation for the reaction of:
i) beryllium with sodium hydroxide solution
ii) Explain your answer in (b) (i)
16. In the manufacture of ammonia, nitrogen is catalytically hydrogenated to give ammonia according to the following equation.
Image
a) i) Name the catalyst used in the reaction
ii) Write the expression for the equilibrium constant, Kp for the reaction.
b) State what would happen to the position of the equilibrium if:
i) pressure was increased
ii) temperature was increased
c) When 3 moles of hydrogen and 1 mole of nitrogen were mixed and allowed to attain equilibrium at 100 atms and 4000C, the equilibrium mixture contained 25% of ammonia by volume.
Calculate the:
i) number of moles of nitrogen and hydrogen at equilibrium
ii) value of the equilibrium constant, Kp at 4000C
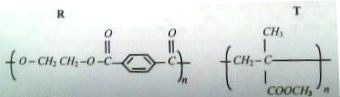
17. a) Differentiate between addition and condensation polymers
b) The structural formulae of two polymers R and T are shown below
Name the polymer;
i) R
ii) T
c) Write the structural formula(e) of monomers(s) of the polymers R and T respectively
d) Give one use of:
i) R
ii) T