1. You are provided with substance Q, which contains two cations and two anions. You are required to carry out tests below on Q and identify the cations and anions in Q.
Identify any gas(es) evolved.
Record your observations and deductions in the table below
|
TESTS |
OBSERVATIONS |
DEDUCTIONS |
|
a) Heat two spatula end-fuls of Q strongly in a dry test tube until there is no further change |
|
|
|
b) To about 6cm3 of water, add 2 spatula end-fuls of Q and shake well. |
|
|
|
c) Divide the filtrate into six parts. i) To the first part of the filtrate, add dilute sodium hydroxide solution dropwise until in excess |
|
|
|
ii) To the second part of the filtrate, add dilute ammonia solution dropwise until in excess |
|
|
|
iii) To the third part of the filtrate, add 2-3 drops of dilute sulphuric acid and heat the mixture. |
|
|
|
iv) Use the forth part of the filtrate to carry a test of your own choice so as to confirm the first cation in Q |
|
|
|
v)To the fifith part of the filtrate add 2-3 drops of lead(II) nitrate solution |
|
|
|
vi) Use the sixth part of the filtrate to carry out a test of your own choice sa as to confirm the first anion |
|
|
|
d) Add dilute nitric acid in small portions to the residue until there is no further change. Divide the resultant solution into three portions. |
|
|
|
i) To the first portion of the solution, add dilute sodium,hydroxide solution dropwise until in excess |
|
|
|
ii) To the second portion of the solution, add dilute ammonia solution dropwise until in excess |
|
|
|
iii) Use the third portion of the solution to carry out a test of your own choice so as to confirm the second cation in Q |
|
Questions:
Identify the
i) cations in Q
ii) anions in Q
3. You are provided with an organic compound W. You are required to carry out tests below in W and describe the nature of W.
Record your observations and deductions in the table below.
|
TESTS |
OBSERVATIONS |
DEDUCTIONS |
|
a) Burn a small amount of W on a spatula end or in a dry porcelain dish. |
|
|
|
b)To about 0.5cm3 of W, add 1cm3 of water, shake and test the mixture with litmus paper |
|
|
|
c) To about 1cm3 of W, add 2-3 drops of neutral iton(III) chloride solution. |
|
|
|
d) To about 1cm3 of W, add 3-4 drops of 2, 4 - dinitrophenylhydrazine |
|
|
|
e) To about 2cm3 of W, add 4-5 drops of acidified potassium dichromate (VI) solution, heat, allow to cool and use in part (f) |
|
|
|
f) To the mixture from €, add 3-4 drops of 2, 4 – dinitrophenylhydrazine |
|
|
|
g) To about 2cm3 of Luca’s reagent, add about 1cm3 of W |
|
|
h) Describe the nature of W.
3. You are provided with the following:
FA1, which is approximately a 0.1M sodium thioslphate solution
FA2, which is a solution containing 2.4g dm-3 of potassium iodate
Solid Y, which is a salt containing dichromate ions
1M sulphuric acid solution
5% potassium iodide solution
Starch solution.
You are required to standardize FA1 and use it to determine the percentage by mass of chromonium in Y.
In acidic solution, iodate and dichromate (VI) ions react with potassium iodide to liberate iodine according to the following equations.
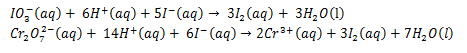
The iodine liberated in both cases reacts with thiosulphate ions according to the following equation
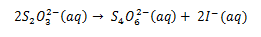
PROCEDURE
a) Pipette 25.0 (or 20.0)cm3 of FA2 into a conical flask and add an equal volume of 1M sulphuric acid using a measuring cylinder, followed by 10cm3 of 5% potassium iodide solution.
Titrate the iodine liberated with FA1, using starch solution as indicator. Repeat the titration until you obtain consistent results.
i) Record your results in Table I below.
Results:
Volume of pipette used………………cm3
Table I
|
Final burette reading (cm3) |
|
|
|
|
Initial burette reading (cm3) |
|
|
|
|
Volume of FA1 used (cm3) |
|
|
|
ii) Volumes of FA1 used for calculating average volume…………..cm3
iii) Average volume of FA1 used…………….cm3
Questions:
a) Calculate the number of moles of iodine liberated by FA2 (O=16; K=39; I= 127)
b) Determine the concentration of FA1 in mol dm-3
PROCEDURE:
b) Weigh accurately about 1.2g of Y. Dissolve it in a minimum amount of distilled water and transfer the solution into a 250cm3 volumetric flask. Make the solution up to the mark with distilled water and label it FA3.
Pipette 25.0 (or 20.0) cm3 of FA3 into a conical flask and add an equal volume of 1M sulphuric acid using a measuring cylinder, followed by 10cm3 of 5% potassium iodide solution.
Titrate the iodine liberated with FA1 using starch as indicator.
Repeat the titration until you obtain consistent results.
i) Record your results in table II below.
RESULTS
Mass of weighing bottle +Y ……….g
Mass of empty weighing bottle……..g
Mass of Y used……………………..g
Volume of pipette used…………….cm3
TABLE II
|
Final burette reading (cm3) |
|
|
|
|
Initial burette reading (cm3) |
|
|
|
|
Volume of FA1 used (cm3) |
|
|
|
ii) Volumes FA1 used for calculating average volume………cm3
iii) Average volume of FA1 used ……………..cm3
Questions:
a) Calculate the number of moles of iodine liberated by FA3
b) Determine the:
i) concentration of FA3 in mol dm-3
ii) mass of chromium in Y and hence its percentage. (Cr = 52)
