SECTION A
1. a) Write the electronic configuration of zinc. (The atomic number of zinc is 30).
b) State
i) two reasons why zinc is not considered as a typical transition element.
ii) one property in which zinc behaves as a transition element.
c) i) Name one ore from which zinc can be extracted and write its formula.
ii) Briefly describe how pure zinc can be obtained from the ore you have named in (c) (i).
d) Describe the reactions of zinc with
i) air.
ii) sodium hydroxide.
(Your answers should include equations for the reactions)
2. a) Describe an experiment that can be carried out to determine the relative molecular mass of benzoic acid in benzene by depression of freezing point method. (Your answer should include a diagram of the apparatus that can be used and the treatment of the results).
b) State four limitations of the depression of freezing point as a method for determination of molecular mass of a substance.
c) A solution containing 0.363g of methanoic acid (HCOOH) in 50g of benzene, froze at 5.0930C. Calculate the molecular mass of methanoic acid. (The freezing point of benzene is 5.5330C; The freezing point constant of benzene is 5.50Cmol-1 kg-1).
d) Comment on your answer in (a)
(The molecular mass of methanoic acid is 46).
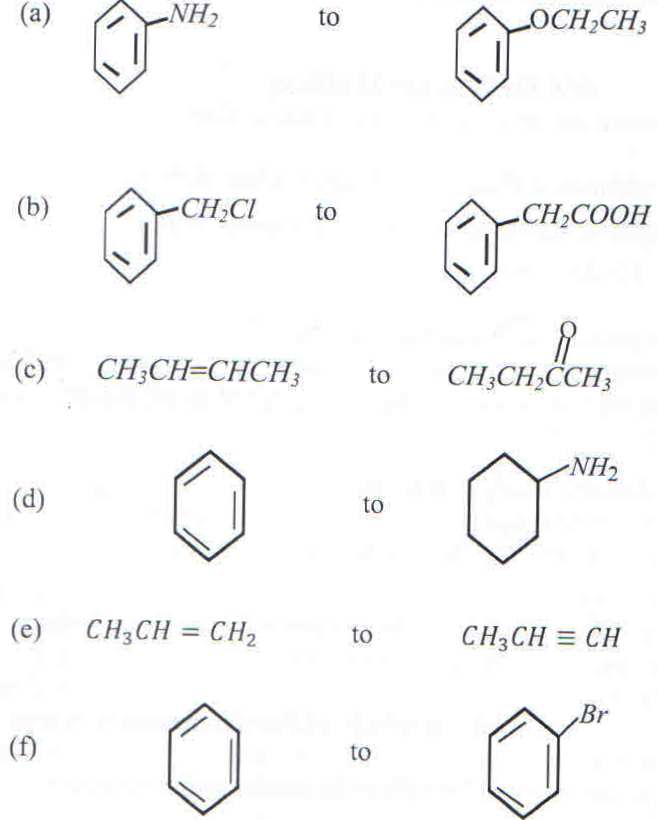
3. Write the equations to show how the following conversions can be carried out. In each case, indicate the reagents and conditions for the reactions.
4a) i)State Kohlrauch’s law of independent migration of ions.
ii) 2.72g of anhydrous zinc chloride was dissolved in water and the solution made up to one litre.The electrolytic conductivity of the solution was fond to be 5.176 × 10-3Ω-1 cm-1 at 250C.
Determine the molar ionic conductivity of chloride ions at this temperature.
(Zn = 65; CL = 35.5; The molar conductivity of zinc chloride at infinite dilution is 106Ω-1 cm2 mol-1).
b) i) Draw sketch graphs to show how molar conductivities of the following compounds vary with concentration.
- Copper (II) Sulphate.
- Hydrofluoric acid.
ii) Explain the shapes of the graphs you have sketched in (b) (i).
c) The table below shows how the molar conductivity of lithium chloride in water at 250C varies with dilution, 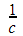 , where c is concentration.
, where c is concentration.
|
Dilution (mo1-1 dm3) |
2000 |
1000 |
500 |
200 |
100 |
20 |
|
Molar conductivity, Λ(Ω-1 cm2 mol-1) |
113.2 |
112.5 |
111.5 |
109.4 |
107.3 |
100.1 |
i) Plot a graph of molar conductivity of lithium chloride against dilution, 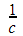 , at 250C.
, at 250C.
ii) Explain the shape of the graph.
iii) Using the graph, estimate the molar conductivity of lithium chloride at infinite dilution at 250C.
SECTION B
5. a) Sulphur trioxide decomposes according to the following equation when heated.
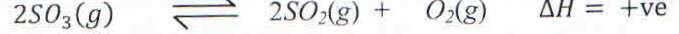
i) State the conditions for the reaction.
ii) Write an expression for the equilibrium constant for the reaction, Kp.
b) When sulphur trioxide was heated in a closed vessel, at 270C and 0.25 atmospheres, 46% of sulphur trioxide was decomposed at equilibrium. Calculate the value of Kp.
c) Calculate the heat of the decomposition of sulphur trioxide. (The heat of formation of sulphur dioxide and sulphur trioxide are -297kJmol-1 and -392kJmol-1 respectively).
d) Explain how the position of the equilibrium, value of the equilibrium constant and the rate of attainment of the equilibrium would be affected if
i) the temperature of the reaction was increased.
ii) the pressure of the reaction was decreased.
iii) a catalyst was added to the reaction mixture.
6. Explain the following observations. (Write equations where neccessary)
a) When ammonia solution is added to a solution containing aluminum ions, a white precipitate is formed, whereas in the presence of ammonium chloride no precipitate is formed.
b) Methyl orange indicator, pH range 3.6 to 4.4, is a suitable indicator in titration of ammonia solution with hydrochloric acid but not suitable for titration of sodium hydroxide solution with Ethanoic acid.
c) When ammonium sulphate is dissolved in water, the solution turns litmus red where as a solution of sodium sulphate has no effect on litmus.
d) The boiling point of ethanol is much higher than that of methoxymethane (diethyl ether) although both have the same molecular mass.
e) When acidified potassium dichromate (VI) solution is added to aqueous solution of potassium iodide, the solution turns brown.
f) When Alpha particles are directed at a thin gold foil, most of them pass through undeflected.
7. The atomic numbers and the boiling points of elements in period 3 of the Periodic Table are shown below.
|
Element |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
Ar |
|
Atomic number |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|
Boiling point (K) |
1250 |
1400 |
2750 |
2900 |
500 |
550 |
400 |
0 |
a) i) Plot a graph of boiling against atomic number.
ii) Explain the shape of the graph you have drawn in (a) (i).
b) Describe how magnesium, aluminum and silicon react with
i) hydrochloric acid
ii) sodium hydroxide
iii) water
(Your answer should include equations for the relevant reactions).
8. a) An organic compound P contains 35.04% Carbon, 6.56% hydrogen and 58.40% bromine. Calculate the empirical formula of P.
(H =1, C =12; Br =79.9)
b) The vapour pressure of P was found to be 68.
i) Determine the molecular formula of P.
ii) Write the names and structural formulae of all the possible isomers of P.
c) When P was reacted with sodium hydroxide, a compound, Q was formed. When a solution of anhydrous zinc chloride in concentrated hydrochloric acid was added to Q, it turned cloudy within 10 minutes.
i) Identify Q.
ii) Write the mechanism for the reaction between P and sodium hydroxide and state the condition(s) for the reaction to take place.
d) Explain the difference in the reactivity of P and bromobenzene with sodium hydroxide.
e) Write equation(s) to show how P can be synthesized from butan-l-ol.
END
