1. A hydrocarbon Z, with molecular formula CxHy, reacts with oxygen according to the following equation:
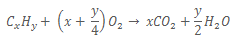
When 20cm3 of Z was exploded in 200cm3 of an excess amount of oxygen, it burnt completely with a sooty flame. The volume of residual gas after cooling to room temperature was 160cm3. When aqueous potassium hydroxide was added, the volume of the gas that finally remained was 20cm3.
a) Calculate the molecular formula of Z
b) When Z was treated with bromine in the presence of iron (III) bromide, the bromine was decolorized. Identify Z.
c) Write equation(s) to show how Z can be synthesized from ethyne
2. a) Complete the following nuclear reaction equations
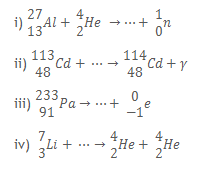
3. State what would be observed and write equation(s) for the reaction(s) that would take place to a solution of iron (II) sulphate when;
a) aqueous sodium hydroxide was added drop-wise until in excess and the mixture was allowed to stand
b) a few drops of concentrated sulphuric acid was added followed by concentrated nitric acid and the mixture was boiled.
4. The data in the Table below was obtained for the reaction between an alkylhaide, R and sodium hydroxide solution.
|
Experiment |
[R] (mol dm-3) |
[OH] (mol dm-3) |
Rate (mol dm-3s-1) |
|
1 2 3 4 |
0.100 0.100 0.50 0.025 |
0.50 0.25 0.25 0.25 |
2.0 x 10-3 2.0 x10-3 1.0 x 10 -3 5.0 x 10 -4 |
a) Determine the order of the reaction with respect to:
i) Alkylhalide, R
ii) Sodium hydroxide
b) Write the rate equation for the reaction
c) i) State the class of alkylhaide
ii) Give a reason for your answer in (c) (i)
5. a) Perspex is a synthetic polymer with a structure
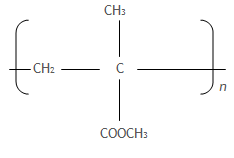
image
i) Name the type of reaction that leads to the formation of Perspex
ii) Write the structure of the monomer of Perspex
b) When 1.25 x 10-3 moles of Perspex were heated strongly with silicon(IV) oxide as a catalyst, 4.85g of the monomer was produced.
Calculate the;
i) value of n
ii) molar mass of Perspex
b) State one use of Perspex.
6.a) When manganese(II) nitrate was heated, a black solid R, was formed.
Write equation for the reaction that took place.
b) R was heated with excess potassium hydroxide:
i) State what was observed
ii) Write equation for the reaction that took place.
c) To the mixture in (b), chlorine gas was bubbled. Write equation for the reaction that took place.
7. The standard electrode potentials for some redox systems are shown below
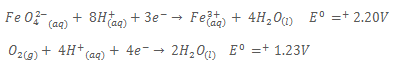
a) Write:
i) The cell notation for the cell formed when the half cells are combined
ii) The overall equation for the reaction
b) i) Calculate the e.m.f of the cell in (a)
ii) State whether the cell reaction in (a) (ii) is feasible or not. Give a reason for your answer.
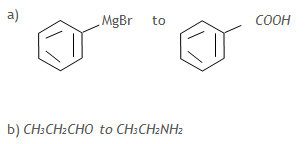
8. Write equation(s) to show how the following conversions can be effected:
9. a) Write equation for the reaction between fluorine and;
i) water
ii) hot concentrated sodium hydroxide
b) Although fluorine is an element in group (VII) of the Periodic Table, it behaves differently from the other members of the group.
State three reasons why fluorine behaves differently from the other members
10. Complete the following equations and in each case outline a mechanism for the reaction.
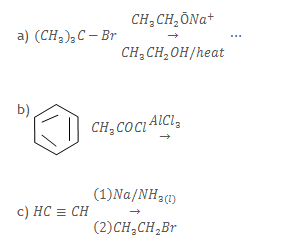
11. Table below shows acid dissociation constants, Ka, for some acids at 250C
|
Acid |
Ka (mol dm-3) |
|
H – COH CH3COOH CH3CH2COOH |
1.70 x 10 -4 1.70 x 10 -5 1.35 x 10-5 |
i)State the trend in acid strength of the acids
ii) Explain your answer
b) Calculate the pH of a 0.5M CH3CH2COOH solution
c) i) 45.0cm3 of a solution in (b) was mixed with 35.0cm3 of a 0.5M potassium hydroxide solution. Calculate the change in pH of the solution
ii) Predict the effect of adding two drops of dilute hydrochloric acid to the solution in (c) (i)
12. The atomic number of aluminum is 13
Write the;
i) electronic configuration of aluminum
ii)formula of the chloride of aluminum
b) Write equation for the reaction between aluminum chloride and
i) water
ii) excess ammonia solution
iii) excess sodium hydroxide solution
c) Name one reagent that can be used to distinguish between aluminum and lead(II)ions in solution
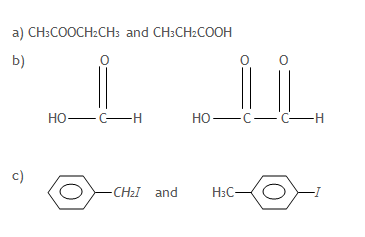
13. Name one reagent that can be used to distinguish between the following pairs of compounds. In each case, state what would be observed if each member of the pair separately treated with the reagent you have named
14. a) Define the term molar conductivity
b) i) Sketch a graph to show the variation of molar conductivity of sodium chloride with dilution
ii) Explain the shape of the graph in (b) (i)
c) The electrolytic conductivity of a saturated solution of silver chloride at 250C is 1.5 x 10-4 Ω-1 m-1. The molar conductivities at infinite dilution of silver and chloride ions are 6.2 x 10-3 and 7.7 x 10-3 Ω-1 m2mol-1 respectively.
Determine the solubility of silver chloride at 250C.
15. Cobalt (II)nitrate dissolves in water to form a pink solution and decomposes on heating to form a green solid.
a) Write equation to show the effect of heat in cobalt (II) nitrate
b) State what would be observed and write equation for the reaction that would take place when the following substances are added to the solution of cobalt (II)nitrate in water
i) Concentrated hydrochloric acid
ii) Aqueous ammonium thiocyanate solution
iii) Aqueous sodium hydroxide
16. a) i) Draw the structure and name the shape of the following oxyanions

ii) Explain the structure of the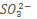 ion
ion
b) i) Name the reagent(s) that can be used to distinguish between the oxyanions in (a) (i)
ii) State what would be observed; if a solution of each of the oxyanion is treated separately with the reagent(s) you have named in (b) (i)
iii) Write the equation(s) for any reaction(s) that would take place when a solution of each of the oxyanions is treated separately with the reagent(s) you have named in (b) (i)
17. a) State three conditions that enable isolation of a solute from a mixture by solvent extraction
b) When one liter of an aqueous solution containing 25.0g of solute X was shaken with 500cm3 of ethoxyethane, 9.7g of X was extracted in the ethoxyethane layer.
Calculate the partition coefficient of X between ethoxyethane and water.
c) The solution in (b) was shaken with two successive 250cm3 portions of ethoxyene. Calculate the total mass extracted.
d) Comment on the result in (c)
e) State one application of solvent extraction
